Pressure RElieving Support SUrfaces: a Randomised Evaluation 2 (PRESSURE 2): study protocol for a randomised controlled trial
- PMID: 27993145
- PMCID: PMC5168811
- DOI: 10.1186/s13063-016-1703-8
Pressure RElieving Support SUrfaces: a Randomised Evaluation 2 (PRESSURE 2): study protocol for a randomised controlled trial
Abstract
Background: Pressure ulcers represent a major burden to patients, carers and the healthcare system, affecting approximately 1 in 17 hospital and 1 in 20 community patients. They impact greatly on an individual's functional status and health-related quality of life. The mainstay of pressure ulcer prevention practice is the provision of pressure redistribution support surfaces and patient repositioning. The aim of the PRESSURE 2 study is to compare the two main mattress types utilised within the NHS: high-specification foam and alternating pressure mattresses, in the prevention of pressure ulcers.
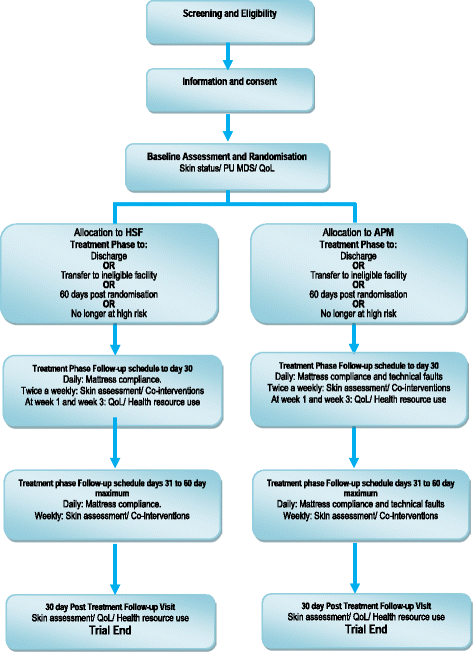
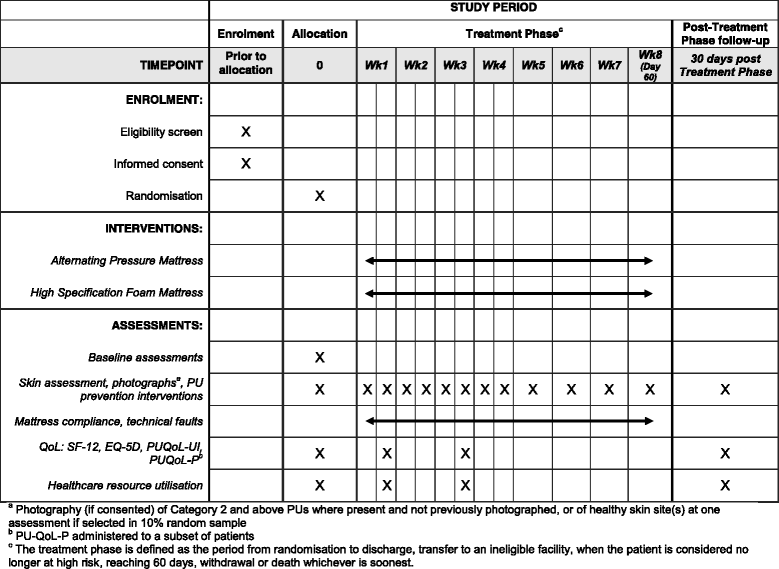
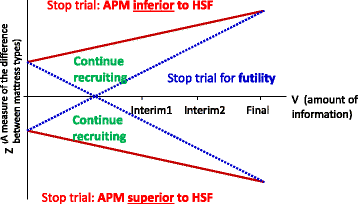
Methods/design: PRESSURE 2 is a multicentre, open-label, randomised, double triangular, group sequential, parallel group trial. A maximum of 2954 'high-risk' patients with evidence of acute illness will be randomised on a 1:1 basis to receive either a high-specification foam mattress or alternating-pressure mattress in conjunction with an electric profiling bed frame. The primary objective of the trial is to compare mattresses in terms of the time to developing a new Category 2 or above pressure ulcer by 30 days post end of treatment phase. Secondary endpoints include time to developing new Category 1 and 3 or above pressure ulcers, time to healing of pre-existing Category 2 pressure ulcers, health-related quality of life, cost-effectiveness, incidence of mattress change and safety. Validation objectives are to determine the responsiveness of the Pressure Ulcer Quality of Life-Prevention instrument and the feasibility of having a blinded endpoint assessment using photography. The trial will have a maximum of three planned analyses with unequally spaced reviews at event-driven coherent cut-points. The futility boundaries are constructed as non-binding to allow a decision for stopping early to be overruled by the Data Monitoring and Ethics Committee.
Discussion: The double triangular, group sequential design of the PRESSURE 2 trial will provide an efficient design through the possibility of early stopping for demonstrating either superiority, inferiority of mattresses or futility of the trial. The trial optimises the potential for producing robust clinical evidence on the effectiveness of two commonly used mattresses in clinical practice earlier than in a conventional design.
Trial registration: ISRCTN01151335 . Registered on 14 May 2013. Protocol version: 5.0, dated 25 September 2015 Trial sponsor: Clare Skinner, Faculty Head of Research Support, University of Leeds, Leeds, LS2 9JT; 0113 343 4897; C.E.Skinner@leeds.ac.uk.
Keywords: Alternating pressure mattress; Double triangular group sequential design; Pressure ulcers; Quality of life; Randomised controlled trial; Standard foam mattress.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical