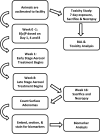
Safety and Preclinical Efficacy of Aerosol Pioglitazone on Lung Adenoma Prevention in A/J Mice
- PMID: 27993834
- PMCID: PMC5687250
- DOI: 10.1158/1940-6207.CAPR-16-0174
Safety and Preclinical Efficacy of Aerosol Pioglitazone on Lung Adenoma Prevention in A/J Mice
Abstract
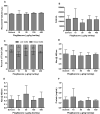
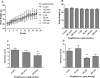
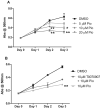
Pioglitazone is a PPARγ agonist commonly prescribed for the clinical treatment of diabetes. We sought to expand its use to lung cancer prevention in a benzo[a]pyrene (B[a]P) mouse model with direct lung delivery via inhalation. Initially, we conducted inhalational toxicity experiments with 0, 15, 50, 150, and 450 μg/kg body weight/day pioglitazone in 40 A/J mice. We examined the animals for any physical toxicity and bronchoalveolar lavage fluids for inflammatory and cytotoxicity markers. Doses up to and including 450 μg/kg bw/d failed to demonstrate toxicity with aerosol pioglitazone. For chemoprevention experiments, A/J mice were randomized to treatment groups of inhaled doses of 0, 50, 150, or 450 μg/kg bw/d pioglitazone 1 or 8 weeks after the last dose of B[a]P. For the early treatment group, we found up to 32% decrease in lung adenoma formation with 450 μg/kg bw/d pioglitazone. We repeated the treatments in a second late-stage experiment and found up to 44% decreases in lung adenoma formation in doses of pioglitazone of 150 and 450 μg/kg bw/day. Both the early- and the late-stage experiments demonstrated biologically relevant and statistically significant decreases in adenoma formation. We conclude that aerosol pioglitazone is well-tolerated in the A/J mouse model and a promising chemoprevention agent for the lower respiratory tract. Cancer Prev Res; 10(2); 124-32. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
Figures
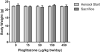
Similar articles
-
Fixed-Dose Combinations of Pioglitazone and Metformin for Lung Cancer Prevention.Cancer Prev Res (Phila). 2017 Feb;10(2):116-123. doi: 10.1158/1940-6207.CAPR-16-0232. Epub 2017 Jan 4. Cancer Prev Res (Phila). 2017. PMID: 28052934 Free PMC article.
-
Chemoprevention of lung carcinogenesis by the combination of aerosolized budesonide and oral pioglitazone in A/J mice.Mol Carcinog. 2011 Dec;50(12):913-21. doi: 10.1002/mc.20751. Epub 2011 Mar 3. Mol Carcinog. 2011. PMID: 21374736 Free PMC article.
-
Chemoprevention of pulmonary carcinogenesis by aerosolized budesonide in female A/J mice.Cancer Res. 1997 Dec 15;57(24):5489-92. Cancer Res. 1997. PMID: 9407956
-
NTP Research Report on the CLARITY-BPA Core Study: A Perinatal and Chronic Extended-Dose-Range Study of Bisphenol A in Rats: Research Report 9 [Internet].Research Triangle Park (NC): National Toxicology Program; 2018 Sep. Research Triangle Park (NC): National Toxicology Program; 2018 Sep. PMID: 31305969 Free Books & Documents. Review.
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
Cited by
-
Inhaled Medicines for Targeting Non-Small Cell Lung Cancer.Pharmaceutics. 2023 Dec 14;15(12):2777. doi: 10.3390/pharmaceutics15122777. Pharmaceutics. 2023. PMID: 38140117 Free PMC article. Review.
-
Effects of PPAR-γ agonists on oral cancer cell lines: Potential horizons for chemopreventives and adjunctive therapies.Head Neck. 2020 Sep;42(9):2542-2554. doi: 10.1002/hed.26286. Epub 2020 Jun 9. Head Neck. 2020. PMID: 32519370 Free PMC article.
-
Intranasal Iloprost Prevents Tumors in a Murine Lung Carcinogenesis Model.Cancer Prev Res (Phila). 2022 Jan;15(1):11-16. doi: 10.1158/1940-6207.CAPR-21-0086. Epub 2021 Sep 23. Cancer Prev Res (Phila). 2022. PMID: 34556494 Free PMC article.
-
Pioglitazone ameliorates retinal ischemia/reperfusion injury via suppressing NLRP3 inflammasome activities.Int J Ophthalmol. 2017 Dec 18;10(12):1812-1818. doi: 10.18240/ijo.2017.12.04. eCollection 2017. Int J Ophthalmol. 2017. PMID: 29259897 Free PMC article.
-
Pioglitazone-mediated reversal of elevated glucose metabolism in the airway epithelium of mouse lung adenocarcinomas.JCI Insight. 2017 Jul 6;2(13):e94220. doi: 10.1172/jci.insight.94220. eCollection 2017 Jul 6. JCI Insight. 2017. PMID: 28679956 Free PMC article.
References
-
- Khuri FR, Lee JJ, Lippman SM, Kim ES, Cooper JS, Benner SE, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98:441–50. - PubMed
-
- Lippman SM, Lee JJ, Martin JW, El-Naggar AK, Xu X, Shin DM, et al. Fenretinide activity in retinoid-resistant oral leukoplakia. Clin Cancer Res. 2006;12:3109–14. - PubMed
-
- Kurie JM, Lotan R, Lee JJ, Lee JS, Morice RC, Liu DD, et al. Treatment of former smokers with 9-cis-retinoic acid reverses loss of retinoic acid receptor-beta expression in the bronchial epithelium: results from a randomized placebo-controlled trial. J Natl Cancer Inst. 2003;95:206–14. - PubMed
-
- Wan H, Hong WK, Lotan R. Increased retinoic acid responsiveness in lung carcinoma cells that are nonresponsive despite the presence of endogenous retinoic acid receptor (RAR) beta by expression of exogenous retinoid receptors retinoid X receptor alpha, RAR alpha, and RAR gamma. Cancer Res. 2001;61:556–64. - PubMed
-
- Khuri FR, Lotan R, Kemp BL, Lippman SM, Wu H, Feng L, et al. Retinoic acid receptor-beta as a prognostic indicator in stage I non-small-cell lung cancer. J Clin Oncol. 2000;18:2798–804. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

