Repurposing Toremifene for Treatment of Oral Bacterial Infections
- PMID: 27993858
- PMCID: PMC5328575
- DOI: 10.1128/AAC.01846-16
Repurposing Toremifene for Treatment of Oral Bacterial Infections
Abstract
The spread of antibiotic resistance and the challenges associated with antiseptics such as chlorhexidine have necessitated a search for new antibacterial agents against oral bacterial pathogens. As a result of failing traditional approaches, drug repurposing has emerged as a novel paradigm to find new antibacterial agents. In this study, we examined the effects of the FDA-approved anticancer agent toremifene against the oral bacteria Porphyromonas gingivalis and Streptococcus mutans We found that the drug was able to inhibit the growth of both pathogens, as well as prevent biofilm formation, at concentrations ranging from 12.5 to 25 μM. Moreover, toremifene was shown to eradicate preformed biofilms at concentrations ranging from 25 to 50 μM. In addition, we found that toremifene prevents P. gingivalis and S. mutans biofilm formation on titanium surfaces. A time-kill study indicated that toremifene is bactericidal against S. mutans Macromolecular synthesis assays revealed that treatment with toremifene does not cause preferential inhibition of DNA, RNA, or protein synthesis pathways, indicating membrane-damaging activity. Biophysical studies using fluorescent probes and fluorescence microscopy further confirmed the membrane-damaging mode of action. Taken together, our results suggest that the anticancer agent toremifene is a suitable candidate for further investigation for the development of new treatment strategies for oral bacterial infections.
Keywords: Porphyromonas gingivalis; Streptococcus mutans; biofilms; oral infections; toremifene.
Copyright © 2017 American Society for Microbiology.
Figures




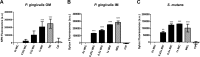



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

