Mechanisms of Acquired Drug Resistance to the HDAC6 Selective Inhibitor Ricolinostat Reveals Rational Drug-Drug Combination with Ibrutinib
- PMID: 27993968
- PMCID: PMC5474138
- DOI: 10.1158/1078-0432.CCR-16-2022
Mechanisms of Acquired Drug Resistance to the HDAC6 Selective Inhibitor Ricolinostat Reveals Rational Drug-Drug Combination with Ibrutinib
Abstract
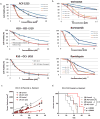
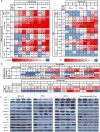
Purpose: Pan-class I/II histone deacetylase (HDAC) inhibitors are effective treatments for select lymphomas. Isoform-selective HDAC inhibitors are emerging as potentially more targeted agents. ACY-1215 (ricolinostat) is a first-in-class selective HDAC6 inhibitor. To better understand the discrete function of HDAC6 and its role in lymphoma, we developed a lymphoma cell line resistant to ACY-1215.Experimental Design: The diffuse large B-cell lymphoma cell line OCI-Ly10 was exposed to increasing concentrations of ACY-1215 over an extended period of time, leading to the development of a resistant cell line. Gene expression profiling (GEP) was performed to investigate differentially expressed genes. Combination studies of ACY-1215 and ibrutinib were performed in cell lines, primary human lymphoma tissue, and a xenograft mouse model.Results: Systematic incremental increases in drug exposure led to the development of distinct resistant cell lines with IC50 values 10- to 20-fold greater than that for parental lines. GEP revealed upregulation of MAPK10, HELIOS, HDAC9, and FYN, as well as downregulation of SH3BP5 and LCK. Gene-set enrichment analysis (GSEA) revealed modulation of the BTK pathway. Ibrutinib was found to be synergistic with ACY-1215 in cell lines as well as in 3 primary patient samples of lymphoma. In vivo confirmation of antitumor synergy was demonstrated with a xenograft of DLBCL.Conclusions: The development of this ACY-1215-resistant cell line has provided valuable insights into the mechanistic role of HDAC6 in lymphoma and offered a novel method to identify rational synergistic drug combinations. Translation of these findings to the clinic is underway. Clin Cancer Res; 23(12); 3084-96. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
J.E.A. and O.A.O. have provided consultancy for Acetylon Pharmaceuticals, Inc
Figures




References
-
- Ning ZQ, et al. Chidamide (CS055/HBI-8000): a new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell-mediated tumor cell cytotoxicity. Cancer Chemotherapy and Pharmacology. 2012;69(4):901–909. - PubMed
-
- San-Miguel JF, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. The Lancet Oncology. 2014;15(11):1195–1206. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

