mTORC1 signaling and primary cilia are required for brain ventricle morphogenesis
- PMID: 27993979
- PMCID: PMC5394754
- DOI: 10.1242/dev.138271
mTORC1 signaling and primary cilia are required for brain ventricle morphogenesis
Abstract
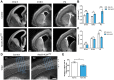
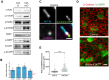
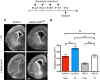
Radial glial cells (RCGs) are self-renewing progenitor cells that give rise to neurons and glia during embryonic development. Throughout neurogenesis, these cells contact the cerebral ventricles and bear a primary cilium. Although the role of the primary cilium in embryonic patterning has been studied, its role in brain ventricular morphogenesis is poorly characterized. Using conditional mutants, we show that the primary cilia of radial glia determine the size of the surface of their ventricular apical domain through regulation of the mTORC1 pathway. In cilium-less mutants, the orientation of the mitotic spindle in radial glia is also significantly perturbed and associated with an increased number of basal progenitors. The enlarged apical domain of RGCs leads to dilatation of the brain ventricles during late embryonic stages (ventriculomegaly), which initiates hydrocephalus during postnatal stages. These phenotypes can all be significantly rescued by treatment with the mTORC1 inhibitor rapamycin. These results suggest that primary cilia regulate ventricle morphogenesis by acting as a brake on the mTORC1 pathway. This opens new avenues for the diagnosis and treatment of hydrocephalus.
Keywords: Cilia; Hydrocephalus; Ventricular system; mTORC1 pathway.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



References
-
- Banizs B., Pike M. M., Millican C. L., Ferguson W. B., Komlosi P., Sheetz J., Bell P. D., Schwiebert E. M. and Yoder B. K. (2005). Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development 132, 5329-5339. 10.1242/dev.02153 - DOI - PubMed
-
- Benadiba C., Magnani D., Niquille M., Morlé L., Valloton D., Nawabi H., Ait-Lounis A., Otsmane B., Reith W., Theil T. et al. (2012). The ciliogenic transcription factor RFX3 regulates early midline distribution of guidepost neurons required for corpus callosum development. PLoS Genet. 8, e1002606 10.1371/journal.pgen.1002606 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

