SMAD3 Regulates Follicle-stimulating Hormone Synthesis by Pituitary Gonadotrope Cells in Vivo
- PMID: 27994055
- PMCID: PMC5313102
- DOI: 10.1074/jbc.M116.759167
SMAD3 Regulates Follicle-stimulating Hormone Synthesis by Pituitary Gonadotrope Cells in Vivo
Abstract
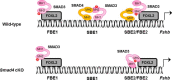
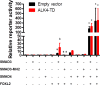
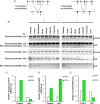
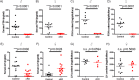
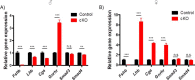
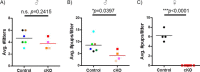
Pituitary follicle-stimulating hormone (FSH) is an essential regulator of fertility in females and of quantitatively normal spermatogenesis in males. Pituitary-derived activins are thought to act as major stimulators of FSH synthesis by gonadotrope cells. In vitro, activins signal via SMAD3, SMAD4, and forkhead box L2 (FOXL2) to regulate transcription of the FSHβ subunit gene (Fshb). Consistent with this model, gonadotrope-specific Smad4 or Foxl2 knock-out mice have greatly reduced FSH and are subfertile. The role of SMAD3 in vivo is unresolved; however, residual FSH production in Smad4 conditional knock-out mice may derive from partial compensation by SMAD3 and its ability to bind DNA in the absence of SMAD4. To test this hypothesis and determine the role of SMAD3 in FSH biosynthesis, we generated mice lacking both the SMAD3 DNA binding domain and SMAD4 specifically in gonadotropes. Conditional knock-out females were hypogonadal, acyclic, and sterile and had thread-like uteri; their ovaries lacked antral follicles and corpora lutea. Knock-out males were fertile but had reduced testis weights and epididymal sperm counts. These phenotypes were consistent with those of Fshb knock-out mice. Indeed, pituitary Fshb mRNA levels were nearly undetectable in both male and female knock-outs. In contrast, gonadotropin-releasing hormone receptor mRNA levels were significantly elevated in knock-outs in both sexes. Interestingly, luteinizing hormone production was altered in a sex-specific fashion. Overall, our analyses demonstrate that SMAD3 is required for FSH synthesis in vivo.
Keywords: SMAD transcription factor; activin; follicle-stimulating hormone (FSH); gene knock-out; pituitary gland.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




Similar articles
-
Follicle-stimulating hormone synthesis and fertility depend on SMAD4 and FOXL2.FASEB J. 2014 Aug;28(8):3396-410. doi: 10.1096/fj.14-249532. Epub 2014 Apr 16. FASEB J. 2014. PMID: 24739304 Free PMC article.
-
Human Follicle-Stimulating Hormone ß Subunit Expression Depends on FOXL2 and SMAD4.Endocrinology. 2020 May 1;161(5):bqaa045. doi: 10.1210/endocr/bqaa045. Endocrinology. 2020. PMID: 32191302 Free PMC article.
-
Conditional Deletion of FOXL2 and SMAD4 in Gonadotropes of Adult Mice Causes Isolated FSH Deficiency.Endocrinology. 2018 Jul 1;159(7):2641-2655. doi: 10.1210/en.2018-00100. Endocrinology. 2018. PMID: 29800110 Free PMC article.
-
Minireview: Activin Signaling in Gonadotropes: What Does the FOX say… to the SMAD?Mol Endocrinol. 2015 Jul;29(7):963-77. doi: 10.1210/me.2015-1004. Epub 2015 May 5. Mol Endocrinol. 2015. PMID: 25942106 Free PMC article. Review.
-
TGF-β Superfamily Regulation of Follicle-Stimulating Hormone Synthesis by Gonadotrope Cells: Is There a Role for Bone Morphogenetic Proteins?Endocrinology. 2019 Mar 1;160(3):675-683. doi: 10.1210/en.2018-01038. Endocrinology. 2019. PMID: 30715256 Free PMC article. Review.
Cited by
-
Decisive gene strategy on osteoarthritis: a comprehensive whole-literature based approach for conclusive gene targets.Aging (Albany NY). 2024 Sep 6;16(17):12346-12378. doi: 10.18632/aging.206094. Epub 2024 Sep 6. Aging (Albany NY). 2024. PMID: 39248710 Free PMC article.
-
Association between SMAD3 gene polymorphisms and osteoarthritis risk: a systematic review and meta-analysis.J Orthop Surg Res. 2018 Sep 12;13(1):232. doi: 10.1186/s13018-018-0939-2. J Orthop Surg Res. 2018. PMID: 30208919 Free PMC article.
-
The Hippo Pathway Effectors YAP and TAZ Regulate LH Release by Pituitary Gonadotrope Cells in Mice.Endocrinology. 2022 Jan 1;163(1):bqab238. doi: 10.1210/endocr/bqab238. Endocrinology. 2022. PMID: 34905605 Free PMC article.
-
Genetic variation of SMAD3 is associated with hip osteoarthritis in a Chinese Han population.J Int Med Res. 2018 Mar;46(3):1178-1186. doi: 10.1177/0300060517745186. Epub 2018 Jan 8. J Int Med Res. 2018. PMID: 29310478 Free PMC article.
-
Betaglycan (TGFBR3) Functions as an Inhibin A, but Not Inhibin B, Coreceptor in Pituitary Gonadotrope Cells in Mice.Endocrinology. 2018 Dec 1;159(12):4077-4091. doi: 10.1210/en.2018-00770. Endocrinology. 2018. PMID: 30364975 Free PMC article.
References
-
- Kumar T. R., Wang Y., Lu N., and Matzuk M. M. (1997) Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat. Genet. 15, 201–204 - PubMed
-
- Matthews C. H., Borgato S., Beck-Peccoz P., Adams M., Tone Y., Gambino G., Casagrande S., Tedeschini G., Benedetti A., and Chatterjee V. K. (1993) Primary amenorrhoea and infertility due to a mutation in the β-subunit of follicle-stimulating hormone. Nat. Genet. 5, 83–86 - PubMed
-
- Simoni M., Gromoll J., and Nieschlag E. (1997) The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr. Rev. 18, 739–773 - PubMed
-
- Wreford N. G., Rajendra Kumar T., Matzuk M. M., and de Kretser D. M. (2001) Analysis of the testicular phenotype of the follicle-stimulating hormone β-subunit knockout and the activin type II receptor knock-out mice by stereological analysis. Endocrinology 142, 2916–2920 - PubMed
-
- Abel M. H., Wootton A. N., Wilkins V., Huhtaniemi I., Knight P. G., and Charlton H. M. (2000) The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology 141, 1795–1803 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

