Dynamic NHERF interaction with TRPC4/5 proteins is required for channel gating by diacylglycerol
- PMID: 27994151
- PMCID: PMC5224389
- DOI: 10.1073/pnas.1612263114
Dynamic NHERF interaction with TRPC4/5 proteins is required for channel gating by diacylglycerol
Abstract
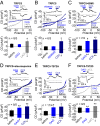
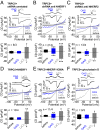
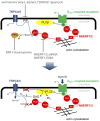
The activation mechanism of the classical transient receptor potential channels TRPC4 and -5 via the Gq/11 protein-phospholipase C (PLC) signaling pathway has remained elusive so far. In contrast to all other TRPC channels, the PLC product diacylglycerol (DAG) is not sufficient for channel activation, whereas TRPC4/5 channel activity is potentiated by phosphatidylinositol 4,5-bisphosphate (PIP2) depletion. As a characteristic structural feature, TRPC4/5 channels contain a C-terminal PDZ-binding motif allowing for binding of the scaffolding proteins Na+/H+ exchanger regulatory factor (NHERF) 1 and 2. PKC inhibition or the exchange of threonine for alanine in the C-terminal PDZ-binding motif conferred DAG sensitivity to the channel. Altogether, we present a DAG-mediated activation mechanism for TRPC4/5 channels tightly regulated by NHERF1/2 interaction. PIP2 depletion evokes a C-terminal conformational change of TRPC5 proteins leading to dynamic dissociation of NHERF1/2 from the C terminus of TRPC5 as a prerequisite for DAG sensitivity. We show that NHERF proteins are direct regulators of ion channel activity and that DAG sensitivity is a distinctive hallmark of TRPC channels.
Keywords: NHERF; PIP2 depletion; TRPC; diacylglycerol; protein interaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Comment in
-
New connections: NHERF gates activity.Sci Signal. 2017 Jan 10;10(461):eaam7242. doi: 10.1126/scisignal.aam7242. Sci Signal. 2017. PMID: 28074011
References
-
- Tai C, Hines DJ, Choi HB, MacVicar BA. Plasma membrane insertion of TRPC5 channels contributes to the cholinergic plateau potential in hippocampal CA1 pyramidal neurons. Hippocampus. 2011;21(9):958–967. - PubMed
-
- Greka A, Navarro B, Oancea E, Duggan A, Clapham DE. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6(8):837–845. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

