Reduced Staphylococcus aureus biofilm formation in the presence of chitosan-coated iron oxide nanoparticles
- PMID: 27994455
- PMCID: PMC5153269
- DOI: 10.2147/IJN.S41371
Reduced Staphylococcus aureus biofilm formation in the presence of chitosan-coated iron oxide nanoparticles
Abstract
Staphylococcus aureus can adhere to most foreign materials and form biofilm on the surface of medical devices. Biofilm infections are difficult to resolve. The goal of this in vitro study was to explore the use of chitosan-coated nanoparticles to prevent biofilm formation. For this purpose, S. aureus was seeded in 96-well plates to incubate with chitosan-coated iron oxide nanoparticles in order to study the efficiency of biofilm formation inhibition. The biofilm bacteria count was determined using the spread plate method; biomass formation was measured using the crystal violet staining method. Confocal laser scanning microscopy and scanning electron microscopy were used to study the biofilm formation. The results showed decreased viable bacteria numbers and biomass formation when incubated with chitosan-coated iron oxide nanoparticles at all test concentrations. Confocal laser scanning microscopy showed increased dead bacteria and thinner biofilm when incubated with nanoparticles at a concentration of 500 µg/mL. Scanning electron microscopy revealed that chitosan-coated iron oxide nanoparticles inhibited biofilm formation in polystyrene plates. Future studies should be performed to study these nanoparticles for anti-infective use.
Keywords: Staphylococcus aureus; biofilm; chitosan-coated iron oxide nanoparticles.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
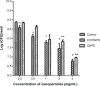
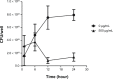



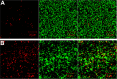

References
-
- Subramani K, Jung RE, Molenberg A, Hammerle CH. Biofilm on dental implants: a review of the literature. Int J Oral Maxillofac Implants. 2009;24(4):616–626. - PubMed
-
- Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty. 2010;25(8):1216–1222. e1–e3. - PubMed
-
- Lellouche J, Kahana E, Elias S, Gedanken A, Banin E. Antibiofilm activity of nanosized magnesium fluoride. Biomaterials. 2009;30(30):5969–5978. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

