A novel combination approach of human polyclonal IVIG and antibiotics against multidrug-resistant Gram-positive bacteria
- PMID: 27994476
- PMCID: PMC5153292
- DOI: 10.2147/IDR.S120227
A novel combination approach of human polyclonal IVIG and antibiotics against multidrug-resistant Gram-positive bacteria
Abstract
Background: Gram-positive bacteria, especially methicillin-resistant Staphylococcus aureus (MRSA) and enterococci, have shown a remarkable ability to develop resistance to antimicrobial agents.
Objective: We aimed to assess possible enhancement of the antimicrobial activity of vancomycin, amoxicillin, clarithromycin, and azithromycin by human polyclonal intravenous immunoglobulin G (IVIG) against 34 multidrug-resistant (MDR) bacterial isolates, including MRSA, Enterococcus faecium, and Enterococcus faecalis.
Materials and methods: Double combinations of the antibiotics with the IVIG were assessed by checkerboard assay, where the interaction was evaluated with respect to the minimum inhibitory concentration (MIC) of the antibiotics. The results of the checkerboard assay were verified in vitro using time-kill assay and in vivo using an invasive sepsis murine model.
Results: The checkerboard assay showed that IVIG enhanced the antimicrobial activity of amoxicillin and clarithromycin against isolates from the three groups of bacteria, which were resistant to the same antibiotics when tested in the absence of IVIG. The efficacy of vancomycin against 15% of the tested isolates was enhanced when it was combined with the antibodies. Antagonism was demonstrated in 47% of the E. faecalis isolates when clarithromycin was combined with the IVIG. Synergism was proved in the time-kill assay when amoxicillin was combined with the antibodies; meanwhile, antagonism was not demonstrated in all tested combinations, even in combinations that showed such response in checkerboard assay.
Conclusion: The suggested approach is promising and could be helpful to enhance the antimicrobial activity of not only effective antibiotics but also antibiotics that have been proven to be ineffective against MDR bacteria. To our knowledge, this combinatorial approach against MDR bacteria, such as MRSA and enterococci, has not been investigated before.
Keywords: Enterococcus faecalis; Enterococcus faecium; MRSA; amoxicillin; human polyclonal IVIG; multidrug resistance; nonconventional antimicrobials; vancomycin.
Conflict of interest statement
The authors declare that none of the submitted materials has any commercial relation and that they do not have conflicts of interest or any source of funding.
Figures
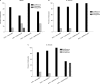


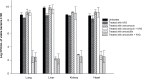
Similar articles
-
A combination of silver nanoparticles and visible blue light enhances the antibacterial efficacy of ineffective antibiotics against methicillin-resistant Staphylococcus aureus (MRSA).Ann Clin Microbiol Antimicrob. 2016 Aug 17;15(1):48. doi: 10.1186/s12941-016-0164-y. Ann Clin Microbiol Antimicrob. 2016. PMID: 27530257 Free PMC article.
-
Antimicrobial activity of tigecycline (GAR-936) against Enterococcus faecium and Staphylococcus aureus used alone and in combination.Pharmacotherapy. 2002 Dec;22(12):1517-23. doi: 10.1592/phco.22.17.1517.34117. Pharmacotherapy. 2002. PMID: 12495161
-
Time-kill kinetics of oritavancin and comparator agents against Staphylococcus aureus, Enterococcus faecalis and Enterococcus faecium.J Antimicrob Chemother. 2009 Jun;63(6):1191-9. doi: 10.1093/jac/dkp126. Epub 2009 Apr 15. J Antimicrob Chemother. 2009. PMID: 19369269
-
Antimicrobial resistance in gram-positive bacteria.Am J Med. 2006 Jun;119(6 Suppl 1):S11-9; discussion S62-70. doi: 10.1016/j.amjmed.2006.03.012. Am J Med. 2006. PMID: 16735146 Review.
-
Treatment options for vancomycin-resistant enterococcal infections.Drugs. 2002;62(3):425-41. doi: 10.2165/00003495-200262030-00002. Drugs. 2002. PMID: 11827558 Review.
Cited by
-
Adjuvant intravenous immunoglobulin administration on postoperative critically ill patients with secondary peritonitis: a retrospective study.Acute Crit Care. 2023 Feb;38(1):21-30. doi: 10.4266/acc.2022.01515. Epub 2023 Feb 27. Acute Crit Care. 2023. PMID: 36935531 Free PMC article.
-
Prevalence of Vancomycin-resistant enterococci (VRE) in Egypt (2010-2022): a systematic review and meta-analysis.J Egypt Public Health Assoc. 2023 Apr 11;98(1):8. doi: 10.1186/s42506-023-00133-9. J Egypt Public Health Assoc. 2023. PMID: 37037955 Free PMC article. Review.
-
Comparison of the clinical characteristics and clinical outcomes of culture-positive septic shock and culture-negative septic shock among pediatric patients.PLoS One. 2023 Jul 14;18(7):e0288615. doi: 10.1371/journal.pone.0288615. eCollection 2023. PLoS One. 2023. PMID: 37450547 Free PMC article.
-
In vitro evaluation of human intravenous immunoglobulin in combination with antimicrobials and human serum against multidrug-resistant isolates of Acinetobacter baumannii.Braz J Microbiol. 2023 Dec;54(4):2845-2856. doi: 10.1007/s42770-023-01153-5. Epub 2023 Oct 31. Braz J Microbiol. 2023. PMID: 37904004 Free PMC article.
-
Polyvalent human immunoglobulin for infectious diseases: Potential to circumvent antimicrobial resistance.Front Immunol. 2023 Jan 9;13:987231. doi: 10.3389/fimmu.2022.987231. eCollection 2022. Front Immunol. 2023. PMID: 36713426 Free PMC article. Review.
References
-
- Carmeli Y, Eliopoulos G, Mozaffari E, Samore M. Health and economic outcomes of vancomycin-resistant enterococci. Arch Intern Med. 2002;162(19):2223–2228. - PubMed
-
- Lodise TP, McKinnon PS. Clinical and economic impact of methicillin resistance in patients with Staphylococcus aureus bacteremia. Diagn Microbiol Infect Dis. 2005;52(2):113–122. - PubMed
-
- Filice GA, Nyman JA, Lexau C, et al. Excess costs and utilization associated with methicillin resistance for patients with Staphylococcus aureus infection. Infect Conrtrol Hosp Epidemiol. 2010;31(4):365–373. - PubMed
-
- Giannakaki V, Miyakis S. Novel antimicrobial agents against multidrug-resistant gram-positive bacteria: an overview. Recent Pat Antiinfect Drug Discov. 2012;7(3):182–188. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

