Cigarette Smoke Induction of Interleukin-27/WSX-1 Regulates the Differentiation of Th1 and Th17 Cells in a Smoking Mouse Model of Emphysema
- PMID: 27994590
- PMCID: PMC5136545
- DOI: 10.3389/fimmu.2016.00553
Cigarette Smoke Induction of Interleukin-27/WSX-1 Regulates the Differentiation of Th1 and Th17 Cells in a Smoking Mouse Model of Emphysema
Abstract
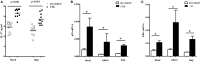
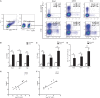
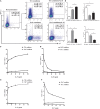
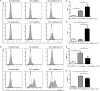
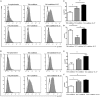
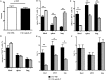
IFN-γ-producing CD4+ T (Th1) cells and IL-17-producing CD4+ T (Th17) cells play a critical role in the pathogenesis of chronic obstructive pulmonary disease (COPD). However, the immune regulation between Th1 and Th17 cells remains unclear. Previous studies have demonstrated that interleukin-27 (IL-27)/WSX-1 exerted pro- or anti-inflammatory effects in many acute inflammatory diseases by modulating T cell-mediated immune response, but little was known about its role in chronic inflammatory disease, especially in smoking-related lung diseases. Considering IL-27 is an important regulator in T lymphocytes immune responses and was found markedly increased in patients with COPD, we hypothesized that IL-27/WSX-1 may exert immuno-regulatory effects on the differentiation of Th1 and Th17 cells in smoking-related COPD. In this study, we aimed to evaluate the expression of IL-27 in patients with COPD and explore the role of IL-27/WSX-1 on Th1 and Th17 cells differentiation in a smoking mouse model of emphysema. We found that elevated expression of IL-27 was associated with increased proportion of Th1 cells and Th17 cells in patients with COPD and demonstrated parallel findings in cigarette smoke-exposed mice. In addition, cigarette smoke exposure upregulated the expression of IL-27R (WSX-1) by naive CD4+ T cells in mice. In vitro, IL-27 significantly augmented the secretion of IFN-γ by naive CD4+ T cells via a T-bet, p-STAT1, and p-STAT3-dependent manner, but inhibited the production of IL-17 by a ROR-γt and p-STAT1-dependent way. Furthermore, anti-IL27 treatment dramatically decreased the expression of IFN-γ-producing CD4+ T cells in cigarette smoke-exposed mice. These findings proposed that IL-27 has functions for promoting the expression of Th1 cells but inhibiting the expression of Th17 cells in vitro and IL-27 neutralization-attenuated Th1-mediated inflammation in vivo, suggesting targeting IL-27/WSX-1 may provide a new therapeutic approach for smoking-related COPD.
Keywords: COPD; IL-27/WSX-1; Th1 cells; Th17 cells; cigarette smoke exposure.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

