Pre-clinical evaluation of a novel class of anti-cancer agents, the Pyrrolo-1, 5-benzoxazepines
- PMID: 27994676
- PMCID: PMC5166549
- DOI: 10.7150/jca.16616
Pre-clinical evaluation of a novel class of anti-cancer agents, the Pyrrolo-1, 5-benzoxazepines
Abstract
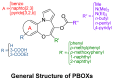
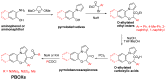
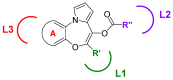
Microtubules are currently ranked one of the most validated targets for chemotherapy; with clinical use of microtubule targeting agents (MTAs) extending beyond half a century. Recent research has focused on the development of novel MTAs to combat drug resistance and drug associated toxicities. Of particular interest are compounds structurally different to those currently used within the clinic. The pyrrolo-1, 5-benzoxazepines (PBOXs) are a structurally distinct novel group of anti-cancer agents, some of which target tubulin. Herein, we review the chemistry, mechanism of action, preclinical development of the PBOXs and comparisons with clinically relevant chemotherapeutics. The PBOXs induce a range of cellular responses including; cell cycle arrest, apoptosis, autophagy, anti-vascular and anti-angiogenic effects. The apoptotic potential of the PBOXs extends across a wide spectrum of cancer-derived cell lines, by targeting tubulin and multiple molecular pathways frequently deregulated in human cancers. Extensive experimental data suggest that combining the PBOXs with established chemotherapeutics or radiation is therapeutically advantageous. Pre-clinical highlights of the PBOXs include; cancer specificity and improved therapeutic efficacy as compared to some current first line therapeutics.
Keywords: 5-benzoxazepines; Pyrrolo-1; apoptosis; drug resistance and G2/M arrest.; tubulin.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures

Similar articles
-
Novel microtubule-targeting agents, pyrrolo-1,5-benzoxazepines, induce apoptosis in multi-drug-resistant cancer cells.Cancer Chemother Pharmacol. 2010 Aug;66(3):585-96. doi: 10.1007/s00280-009-1200-9. Epub 2009 Dec 18. Cancer Chemother Pharmacol. 2010. PMID: 20020128
-
Induction of apoptosis in oral squamous carcinoma cells by pyrrolo-1,5-benzoxazepines.Mol Med Rep. 2015 Sep;12(3):3748-3754. doi: 10.3892/mmr.2015.3832. Epub 2015 May 25. Mol Med Rep. 2015. PMID: 26005189
-
Dual targeting of tumour cells and host endothelial cells by novel microtubule-targeting agents, pyrrolo-1,5-benzoxazepines.Cancer Chemother Pharmacol. 2010 Jan;65(2):289-300. doi: 10.1007/s00280-009-1033-6. Cancer Chemother Pharmacol. 2010. PMID: 19479253
-
Microtubule-targeting agents and their impact on cancer treatment.Eur J Cell Biol. 2020 May;99(4):151075. doi: 10.1016/j.ejcb.2020.151075. Epub 2020 May 1. Eur J Cell Biol. 2020. PMID: 32414588 Review.
-
The Potential of Combining Tubulin-Targeting Anticancer Therapeutics and Immune Therapy.Int J Mol Sci. 2019 Jan 30;20(3):586. doi: 10.3390/ijms20030586. Int J Mol Sci. 2019. PMID: 30704031 Free PMC article. Review.
References
-
- Campiani G, Nacci V, Fiorini I. et al. Synthesis, biological activity, and SARs of pyrrolobenzoxazepine derivatives, a new class of specific "peripheral-type" benzodiazepine receptor ligands. J Med Chem. 1996;39:3435–45. - PubMed
-
- Zisterer DM, Hance N, Campiani G. et al. Antiproliferative action of pyrrolobenzoxazepine derivatives in cultured cells: absence of correlation with binding to the peripheral-type benzodiazepine binding site. Biochem Pharmacol. 1998;55:397–403. - PubMed
-
- Zisterer DM, Campiani G, Nacci V, Williams DC. Pyrrolo-1, 5-benzoxazepines induce apoptosis in HL-60, Jurkat, and Hut-78 cells: a new class of apoptotic agents. J Pharmacol Exp Ther. 2000;293:48–59. - PubMed
-
- Mc Gee MM, Gemma S, Butini S. et al. Pyrrolo[1,5]benzoxa(thia)zepines as a new class of potent apoptotic agents. Biological studies and identification of an intracellular location of their drug target. J Med Chem. 2005;48:4367–77. - PubMed
-
- Mc Gee MM, Campiani G, Ramunno A. et al. Pyrrolo-1, 5-benzoxazepines induce apoptosis in chronic myelogenous leukemia (CML) cells by bypassing the apoptotic suppressor bcr-abl. J Pharmacol Exp Ther. 2001;296:31–40. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

