Stem Cell Monitoring with a Direct or Indirect Labeling Method
- PMID: 27994682
- PMCID: PMC5135688
- DOI: 10.1007/s13139-015-0380-y
Stem Cell Monitoring with a Direct or Indirect Labeling Method
Abstract
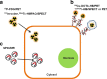
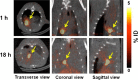
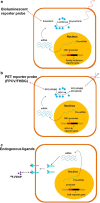
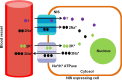
The molecular imaging techniques allow monitoring of the transplanted cells in the same individuals over time, from early localization to the survival, migration, and differentiation. Generally, there are two methods of stem cell labeling: direct and indirect labeling methods. The direct labeling method introduces a labeling agent into the cell, which is stably incorporated or attached to the cells prior to transplantation. Direct labeling of cells with radionuclides is a simple method with relatively fewer adverse events related to genetic responses. However, it can only allow short-term distribution of transplanted cells because of the decreasing imaging signal with radiodecay, according to the physical half-lives, or the signal becomes more diffuse with cell division and dispersion. The indirect labeling method is based on the expression of a reporter gene transduced into the cell before transplantation, which is then visualized upon the injection of an appropriate probe or substrate. In this review, various imaging strategies to monitor the survival and behavior change of transplanted stem cells are covered. Taking these new approaches together, the direct and indirect labeling methods may provide new insights on the roles of in vivo stem cell monitoring, from bench to bedside.
Keywords: Direct labeling method; Indirect labeling method; Radionuclide; Stem cell tracking.
Conflict of interest statement
Min Hwan Kim, Yong Jin Lee, and Joo Hyun Kang declare that they have no conflict of interest, etc. Ethical Statement The care, maintenance, and treatment of animals in these studies followed protocols approved by the Institutional Animal Care and Use Committee of the Korea Institute of Radiological and Medical Sciences (KIRAMS).
Figures


References
-
- Al-Nbaheen M, Vishnubalaji R, Ali D, Bouslimi A, Al-Jassir F, Megges M, et al. Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev. 2013;9:32–43. doi: 10.1007/s12015-012-9365-8. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
