Turning a Substrate Peptide into a Potent Inhibitor for the Histone Methyltransferase SETD8
- PMID: 27994746
- PMCID: PMC5150682
- DOI: 10.1021/acsmedchemlett.6b00303
Turning a Substrate Peptide into a Potent Inhibitor for the Histone Methyltransferase SETD8
Abstract
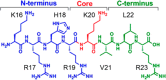
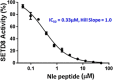
SETD8 is a histone H4-K20 methyltransferase that plays an essential role in the maintenance of genomic integrity during mitosis and in DNA damage repair, making it an intriguing target for cancer research. While some small molecule inhibitors for SETD8 have been reported, the structural binding modes for these inhibitors have not been revealed. Using the complex structure of the substrate peptide bound to SETD8 as a starting point, different natural and unnatural amino acid substitutions were tested, and a potent (Ki 50 nM, IC50 0.33 μM) and selective norleucine containing peptide inhibitor has been obtained.
Keywords: HMT; SETD8; cancer; drug design; structure-based drug design.
Conflict of interest statement
The authors declare the following competing financial interest(s): The Structural Genomics Consortium (authors W.Y., M.V., F.L., and P.J.B.) is a registered charity (number 1097737) that receives funds from AbbVie, Bayer Pharma AG, Boehringer Ingelheim, Canada Foundation for Innovation, Eshelman Institute for Innovation, Genome Canada, Innovative Medicines Initiative (EU/EFPIA), Janssen, Merck & Co., Novartis Pharma AG, Pfizer, So Paulo Research Foundation-FAPESP, Takeda, and Wellcome Trust. R.A.J., H.Z., A.K.U., P.M.B., C.W.H., M.T., V.L.M., W.N.P., C.S., and A.M.P. are employees of AbbVie. The design, study conduct, and financial support for the research of these authors were provided by AbbVie. AbbVie participated in the interpretation of the data, review, and approval of the publication.
Figures


References
-
- Takawa M.; Cho H.-S.; Hayami S.; Toyokawa G.; Kogure M.; Yamane Y.; Iwai Y.; Maejima K.; Ueda K.; Masuda A.; Dohmae N.; Field H. I.; Tsunoda T.; Kobayashi T.; Akasu T.; Sugiyama M.; Ohnuma S.-i.; Atomi Y.; Ponder B. A. J.; Nakamura Y.; Hamamoto R. Histone lysine methyltransferase SETD8 promotes carcinogenesis by deregulating PCNA expression. Cancer Res. 2012, 72, 3217–3227. 10.1158/0008-5472.CAN-11-3701. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

