Discovery of Pyrazolopyrimidine Derivatives as Novel Dual Inhibitors of BTK and PI3Kδ
- PMID: 27994757
- PMCID: PMC5150666
- DOI: 10.1021/acsmedchemlett.6b00356
Discovery of Pyrazolopyrimidine Derivatives as Novel Dual Inhibitors of BTK and PI3Kδ
Abstract
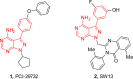
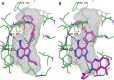
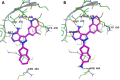
The aberrant activation of B-cells has been implicated in several types of cancers and hematological disorders. BTK and PI3Kδ are kinases responsible for B-cell signal transduction, and inhibitors of these enzymes have demonstrated clinical benefit in certain types of lymphoma. Simultaneous inhibition of these pathways could result in more robust responses or overcome resistance as observed in single agent use. We report a series of novel compounds that have low nanomolar potency against both BTK and PI3Kδ as well as acceptable PK properties that could be useful in the development of treatments against B-cell related diseases.
Keywords: B-cell; BCR; BTK; PI3K; inhibitor; p110δ; pyrazolopyrimidine.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
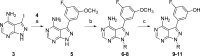

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

