Transition in the mechanism of flow-mediated dilation with aging and development of coronary artery disease
- PMID: 27995364
- PMCID: PMC6758541
- DOI: 10.1007/s00395-016-0594-x
Transition in the mechanism of flow-mediated dilation with aging and development of coronary artery disease
Abstract
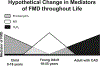
In microvessels of patients with coronary artery disease (CAD), flow-mediated dilation (FMD) is largely dependent upon the endothelium-derived hyperpolarizing factor H2O2. The goal of this study is to examine the influence of age and presence or absence of disease on the mechanism of FMD. Human coronary or adipose arterioles (~150 µm diameter) were prepared for videomicroscopy. The effect of inhibiting COX [indomethacin (Indo) or NOS (L-NAME), eliminating H2O2 (polyethylene glycol-catalase (PEG-CAT)] or targeting a reduction in mitochondrial ROS with scavengers/inhibitors [Vitamin E (mtVitamin E); phenylboronic acid (mtPBA)] was determined in children aged 0-18 years; young adults 19-55 years; older adults >55 years without CAD, and similarly aged adults with CAD. Indo eliminated FMD in children and reduced FMD in younger adults. This response was mediated mainly by PGI2, as the prostacyclin-synthase-inhibitor trans-2-phenyl cyclopropylamine reduced FMD in children and young adults. L-NAME attenuated dilation in children and younger adults and eliminated FMD in older adults without CAD, but had no effect on vessels from those with CAD, where mitochondria-derived H2O2 was the primary mediator. The magnitude of dilation was reduced in older compared to younger adults independent of CAD. Exogenous treatment with a sub-dilator dose of NO blocked FMD in vessels from subjects with CAD, while prolonged inhibition of NOS in young adults resulted in a phenotype similar to that observed in disease. The mediator of coronary arteriolar FMD evolves throughout life from prostacyclin in youth, to NO in adulthood. With the onset of CAD, NO-inhibitable release of H2O2 emerges as the exclusive mediator of FMD. These findings have implications for use of pharmacological agents, such as nonsteroidal anti-inflammatory agents in children and the role of microvascular endothelium in cardiovascular health.
Keywords: Coronary artery disease; Flow-mediated dilation; Microvasculature; Vasodilation.
Figures


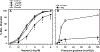


References
-
- Beyer AM, Durand MJ, Hockenberry J, Gamblin TC, Phillips SA, Gutterman DD (2014) An acute rise in intraluminal pressure shifts the mediator of flow-mediated dilation from nitric oxide to hydrogen peroxide in human arterioles. Am J Physiol Heart Circ Physiol 307:H1587–H1593. doi:10.1152/ajpheart.00557.2014 - DOI - PMC - PubMed
-
- Beyer AM, Freed JK, Durand MJ, Riedel M, Ait-Aissa K, Green P, Hockenberry JC, Morgan RG, Donato AJ, Peleg R, Gasparii M, Rokkas CK, Santos JH, Priel E, Gutterman DD (2015) Critical role for telomerase in the mechanism of flow mediated dilation in the human microcirculation. Circ Res. doi:10.1161/circresaha.115.307918 - DOI - PMC - PubMed
-
- Beyer AM, Freed JK, Durand MJ, Riedel M, Ait-Aissa K, Green P, Hockenberry JC, Morgan RG, Donato AJ, Peleg R, Gasparri M, Rokkas CK, Santos JH, Priel E, Gutterman DD (2016) critical role for telomerase in the mechanism of flow-mediated dilation in the human microcirculation. Circ Res 118:856–866. doi:10.1161/CIRCRESAHA.115.307918 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

