Mechanisms of Global Cerebral Edema Formation in Aneurysmal Subarachnoid Hemorrhage
- PMID: 27995510
- PMCID: PMC5336395
- DOI: 10.1007/s12028-016-0354-7
Mechanisms of Global Cerebral Edema Formation in Aneurysmal Subarachnoid Hemorrhage
Abstract
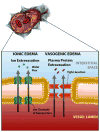
A growing body of clinical literature emphasizes the impact of cerebral edema in early brain injury following aneurysmal subarachnoid hemorrhage (aSAH). Aneurysm rupture itself initiates global cerebral edema in up to two thirds of cases. Although cerebral edema is not a universal feature of aSAH, it portends a poor clinical course, with quantitative analysis revealing a direct correlation between cerebral edema and poor outcome, including mortality and cognitive deficits. Mechanistically, global cerebral edema has been linked to global ischemia at the time of aneurysm rupture, dysfunction of autoregulation, blood breakdown products, neuroinflammation, and hyponatremia/endocrine abnormalities. At a molecular level, several culprits have been identified, including aquaporin-4, matrix metalloproteinase-9, SUR1-TRPM4 cation channels, vascular endothelial growth factor, bradykinin, and others. Here, we review these cellular and molecular mechanisms of global cerebral edema formation in aSAH. Given the importance of edema to the outcome of patients with aSAH and its status as a highly modifiable pathological process, a better understanding of cerebral edema in aSAH promises to hasten the development of medical therapies to improve outcomes in this frequently devastating disease.
Keywords: Edema; Keywords; Subarachnoid hemorrhage.
Conflict of interest statement
Dr. Simard holds a US patent (7,285,574), a novel non-selective cation channel in neural cells and methods for treating brain swelling. Dr. Simard is a member of the scientific advisory board and holds shares in Remedy Pharmaceuticals. No support, direct or indirect, was provided to Dr. Simard, or for this project, by Remedy Pharmaceuticals. All other authors report no conflicts.
Figures
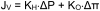
References
-
- Rieth KG, Fujiwara K, Di Chiro G, et al. Serial measurements of CT attenuation and specific gravity in experimental cerebral edema. Radiology. 1980;135:343–8. - PubMed
-
- Na DG, Kim EY, Ryoo JW, et al. CT sign of brain swelling without concomitant parenchymal hypoattenuation: comparison with diffusion- and perfusion-weighted MR imaging. Radiology. 2005;235:992–48. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

