Longchain polyunsaturated fatty acid supplementation in preterm infants
- PMID: 27995607
- PMCID: PMC6463838
- DOI: 10.1002/14651858.CD000375.pub5
Longchain polyunsaturated fatty acid supplementation in preterm infants
Abstract
Background: Controversy exists over whether longchain polyunsaturated fatty acids (LCPUFA) are essential nutrients for preterm infants because they may not be able to synthesise sufficient amounts of LCPUFA to meet the needs of the developing brain and retina.
Objectives: To assess whether supplementation of formula milk with LCPUFA is safe and of benefit to preterm infants. The main areas of interest were the effects of supplementation on the visual function, development and growth of preterm infants.
Search methods: Trials were identified by searching the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 2) in the Cochrane Library (searched 28 February 2016), MEDLINE Ovid (1966 to 28 February 2016), Embase Ovid (1980 to 28 February 2016), CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1980 to 28 February 2016), MEDLINE In Process & Other Non-indexed Citations (1966 to 28 February 2016) and by checking reference lists of articles and conference proceedings. We also searched ClinicalTrials.gov (13 April 2016). No language restrictions were applied.
Selection criteria: All randomised trials evaluating the effect of LCPUFA-supplemented formula in enterally-fed preterm infants (compared with standard formula) on visual development, neurodevelopment and physical growth. Trials reporting only biochemical outcomes were not included.
Data collection and analysis: All authors assessed eligibility and trial quality, two authors extracted data separately. Study authors were contacted for additional information.
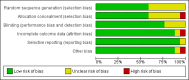
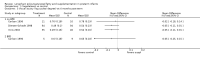
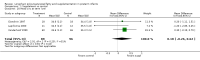
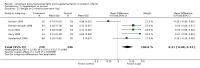
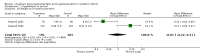
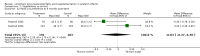
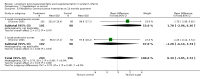
Main results: Seventeen trials involving 2260 preterm infants were included in the review. The risk of bias varied across the included trials with 10 studies having low risk of bias in a majority of the domains. The median gestational age (GA) in the included trials was 30 weeks and median birth weight (BW) was 1300 g. The median concentration of docosahexaenoic acid (DHA) was 0.33% (range: 0.15% to 1%) and arachidonic acid (AA) 0.37% (range: 0.02% to 0.84%). Visual acuity Visual acuity over the first year was measured by Teller or Lea acuity cards in eight studies, by visual evoked potential (VEP) in six studies and by electroretinogram (ERG) in two studies. Most studies found no significant differences in visual acuity between supplemented and control infants. The form of data presentation and the varying assessment methods precluded the use of meta-analysis. A GRADE analysis for this outcome indicated that the overall quality of evidence was low. Neurodevelopment Three out of seven studies reported some benefit of LCPUFA on neurodevelopment at different postnatal ages. Meta-analysis of four studies evaluating Bayley Scales of Infant Development at 12 months (N = 364) showed no significant effect of supplementation (Mental Development Index (MDI): MD 0.96, 95% CI -1.42 to 3.34; P = 0.43; I² = 71% - Psychomotor DeveIopment Index (PDI): MD 0.23, 95% CI -2.77 to 3.22; P = 0.88; I² = 81%). Furthermore, three studies at 18 months (N = 494) also revealed no significant effect of LCPUFA on neurodevelopment (MDI: MD 2.40, 95% CI -0.33 to 5.12; P = 0.08; I² = 0% - PDI: MD 0.74, 95% CI -1.90 to 3.37; P = 0.58; I² = 54%). A GRADE analysis for these outcomes indicated that the overall quality of evidence was low. Physical growth Four out of 15 studies reported benefits of LCPUFA on growth of supplemented infants at different postmenstrual ages (PMAs), whereas two trials suggested that LCPUFA-supplemented infants grow less well. One trial reported mild reductions in length and weight z scores at 18 months. Meta-analysis of five studies (N = 297) showed increased weight and length at two months post-term in supplemented infants (Weight: MD 0.21, 95% CI 0.08 to 0.33; P = 0.0010; I² = 69% - Length: MD 0.47, 95% CI 0.00 to 0.94; P = 0.05; I² = 0%). Meta-analysis of four studies at a corrected age of 12 months (N = 271) showed no significant effect of supplementation on growth outcomes (Weight: MD -0.10, 95% CI -0.31 to 0.12; P = 0.34; I² = 65% - Length: MD 0.25; 95% CI -0.33 to 0.84; P = 0.40; I² = 71% - Head circumference: MD -0.15, 95% CI -0.53 to 0.23; P = 0.45; I² = 0%). No significant effect of LCPUFA on weight, length or head circumference was observed on meta-analysis of two studies (n = 396 infants) at 18 months (Weight: MD -0.14, 95% CI -0.39 to 0.10; P = 0.26; I² = 66% - Length: MD -0.28, 95% CI -0.91 to 0.35; P = 0.38; I² = 90% - Head circumference: MD -0.18, 95% CI -0.53 to 0.18; P = 0.32; I² = 0%). A GRADE analysis for this outcome indicated that the overall quality of evidence was low.
Authors' conclusions: Infants enrolled in the trials were relatively mature and healthy preterm infants. Assessment schedule and methodology, dose and source of supplementation and fatty acid composition of the control formula varied between trials. On pooling of results, no clear long-term benefits or harms were demonstrated for preterm infants receiving LCPUFA-supplemented formula.
Conflict of interest statement
None.
Figures
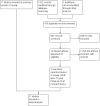



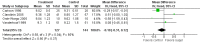








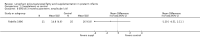
























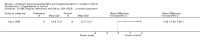
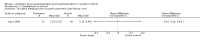



















Update of
-
Long-chain polyunsaturated fatty acid supplementation in preterm infants.Cochrane Database Syst Rev. 2011 Feb 16;(2):CD000375. doi: 10.1002/14651858.CD000375.pub4. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2016 Dec 20;12:CD000375. doi: 10.1002/14651858.CD000375.pub5. PMID: 21328248 Updated.
References
References to studies included in this review
Carlson 1992 {published and unpublished data}
-
- Carlson SE. Lipid requirements of VLBW infants for optimal growth and development. Lipids, Learning and the Brain: Fats in infant formula. Report of the 103rd Ross Conference on Pediatric Research. Columbus, Ohio: Ross Labs, 1993.
-
- Carlson SE, Cooke RJ, Rhodes PG, Peeples JM, Werkman SH, Tolley EA. Longterm feeding of formulas high in LA and marine oil to VLBW infants: phospholipid fatty acids. Pediatric Research 1991;30(5):404‐12. - PubMed
-
- Carlson SE, Cooke RJ, Werkman SH, Tolley EA. First year growth of infants fed standard formula compared with marine oil supplemented formula. Lipids 1992;27(11):901‐7. - PubMed
-
- Carlson SE, Werkman SH, Rhodes PG, Tolley EA. Visual acuity development in healthy preterm infants: effect of marine oil supplementation. American Journal of Clinical Nutrition 1993;58(1):35‐42. - PubMed
Carlson 1996 {published data only}
-
- Carlson SE, Werkman SH. A randomised trial of visual attention of preterm infants fed DHA until 2 months. Lipids 1996;31(1):85‐90. - PubMed
-
- Carlson SE, Werkman SH, Tolley EA. Effect of long chain n‐3 fatty acid supplementation on visual acuity and growth of preterm infants with and without bronchopulmonary dysplasia. American Journal of Clinical Nutrition 1996;63(5):687‐97. - PubMed
Carnielli 2007 {published data only}
-
- Carnielli VP, Simonato M, Verlato G, Luijendijk I, Curtis M, Sauer PJJ, et al. Synthesis of long‐chain polyunsaturated fatty acids in preterm newborns fed formula with long‐chain polyunsaturated fatty acids. American Journal of Clinical Nutrition 2007;86(5):1323‐30. - PubMed
Clandinin 1997 {published data only}
-
- Clandinin MT, Aerde JE, Parrott A, Field CJ, Euler AR, Lien EL. Assessment of the efficacious dose of arachidonic and docosahexaenoic acids in preterm infant formulas: fatty acid composition of erythrocyte membrane lipids. Pediatric Research 1997;42(6):819‐25. - PubMed
Clandinin 2005 {published data only}
-
- Clandinin MT, Aerde JE, Merkel KL, Harris CL, Springer MA, Hansen JW, et al. Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. Journal of Pediatrics 2005;146(4):461‐8. - PubMed
Diersen‐Schade 1998 {published data only}
-
- Diersen‐Schade DA, Hansen JW, Harris CL, Merkel KL, Wisont KD, Boettcher JA. Docosahexaenoic acid plus arachidonic acid enhance preterm infant growth. In: Riemersma RA, Armstrong R, Kelly RW, Wilson R editor(s). Essential Fatty Acids & Eicosanoids: Invited Papers from the Fourth International Congress. Champaign, IL: AOCS Press, 1998:123‐7.
Fadella 1996 {published data only}
Fang 2005 {published data only}
-
- Fang PC, Kuo HK, Huang CB, Ko TY, Chen CC, Chung MY. The effect of supplementation of docosahexaenoic acid and arachidonic acid on visual acuity and neurodevelopment in larger preterm infants. Chang Gung Medical Journal 2005;28(10):708‐15. - PubMed
Fewtrell 2002 {published data only}
-
- Fewtrell MS, Morley R, Abbott RA, Singhal A, Isaacs EB, Stephenson T, MacFayden U, Lucas A. Double‐blind, randomised trial of long‐chain polyunsaturated fatty acids in formula fed to preterm infants. Pediatrics 2002;110(1 Pt 1):73‐82. - PubMed
Fewtrell 2004 {published data only}
-
- Fewtrell MS, Abbott RA, Kennedy K, Singhal A, Morley R, Caine E, et al. Randomized, double‐blind trial of long‐chain polyunsaturated fatty acid supplementation with fish oil and borage oil in preterm infants. Journal of Pediatrics 2004;144(4):471‐9. - PubMed
-
- Isaacs EB, Ross S, Kennedy K, Weaver LT, Lucas A, Fewtrell MS. 10‐year cognition in preterms after random assignment to fatty acid supplementation in infancy. Pediatrics 2011;128(4):e890‐8. - PubMed
-
- Kennedy K, Ross S, Isaacs EB, Weaver LT, Singhal A, Lucas A, et al. The 10‐year follow‐up of a randomised trial of long‐chain polyunsaturated fatty acid supplementation in preterm infants: effects on growth and blood pressure. Archives of Disease in Childhood 2010;95(8):588‐95. - PubMed
Groh‐Wargo 2005 {published data only}
-
- Groh‐Wargo S, Jacobs J, Auestad N, O'Connor DL, Moore JJ, Lerner E. Body composition in preterm infants who are fed long‐chain polyunsaturated fatty acids: A prospective, randomized, controlled trial. Pediatric Research 2005;57(5 Pt 1):712‐8. - PubMed
Innis 2002 {published data only}
-
- Innis SM, Adamkin DH, Hall RT, Kalhan SC, Lair C, Lim M, et al. Docosahexanoic acid and arachidonic acid enhance growth with no adverse effects in preterm infants fed formula. Journal of Pediatrics 2002;140(5):547‐54. - PubMed
Lapillonne 2000 {published data only}
-
- Lapillonne A, Picaud JC, Chirouze V, Goudable J, Reygrobellet B, Claris O, et al. The use of low‐EPA fish oil for long‐chain polyunsaturated fatty acid supplementation of preterm infants. Pediatric Research 2000;48(6):835‐41. - PubMed
O'Connor 2001 {published data only}
-
- O'Connor DL, Hall R, Adamkin D, Austead N, Castillo M, Connor WE, et al. Growth and development in preterm infants fed longchain polyunsaturated fatty acids: A prospective randomised trial. Pediatrics 2001;108(2):359‐71. - PubMed
Uauy 1990 {published data only}
-
- Birch DG, Birch EE, Hoffman DR, Uauy RD. Retinal development of very low birthweight infants fed diets differing in n‐3 fatty acids. Investigative Ophthalmology & Visual Science 1992;33(8):2365‐76. - PubMed
-
- Hoffman DR, Uauy R. Essentiality of dietary n‐3 fatty acids for premature infants; plasma and red blood cell fatty acid composition. Lipids 1992;27(11):886‐95. - PubMed
-
- Uauy R, Hoffman DR, Birch EE, Birch DG, Jameson DM, Tyson J. Safety and efficacy of n‐3 fatty acids in the nutrition of very low birthweight infants: soy oil and marine oil supplementation of formula. Journal of Pediatrics 1994;124(4):612‐20. - PubMed
-
- Uauy RD, Birch DG, Birch EE, Tyson JE, Hoffman DR. Effect of dietary omega 3 fatty acids on retinal function of very low birthweight neonates. Pediatric Research 1990;28:485‐92. - PubMed
Vanderhoof 1999 {published data only}
-
- Vanderhoof J, Gross S, Hegyi T. A multicenter long‐term safety and efficacy trial of preterm formula supplemented with long‐chain polyunsaturated fatty acids. Journal of Pediatric Gastroenterology and Nutrition 2000;31(2):121‐7. - PubMed
-
- Vanderhoof J, Gross S, Hegyi T, Clandinin T, Porcelli P, DeCristofaro J, et al. Evaluation of a long‐chain polyunsaturated fatty acid supplemented formula on growth, tolerance, and plasma lipids in preterm infants up to 48 weeks postconceptional age. Journal of Pediatric Gastroenterology and Nutrition 1999;29(3):318‐26. - PubMed
van Wezel 2002 {published data only}
-
- Wezel‐Meijler G, Knaap MS, Huisman J, Jonkman EJ, Valk J, Lafeber HN. Dietary supplementation of long‐chain polyunsaturated fatty acids in preterm infants: effects on cerebral maturation. Acta Paediatrica 2002;91(9):942‐50. - PubMed
References to studies excluded from this review
Almaas 2015 {published data only}
-
- Almaas AN, Tamnes CK, Nakstad B, Henriksen C, Walhovd KB, Fjell AM, et al. Long‐chain polyunsaturated fatty acids and cognition in VLBW infants at 8 years: an RCT. Pediatrics 2015;135(6):972‐80. - PubMed
Alshweki 2015 {published data only}
Baack 2016 {published data only}
Collins 2010 {published data only}
-
- Collins CT, Makride M, Gibson RA, Ryan P, McPhee AJ. Growth outcomes from the DINO (DHA for the improvement of neurodevelopmental outcome in preterm infants) trial. Journal of Paediatric and Child Health. 2010; Vol. 46:76.
Collins 2011 {published data only}
-
- Collins CT, Makrides M, Gibson RA, McPhee AJ, Davids PG, Doyle LW, et al. Pre‐ and post‐term growth in pre‐term infants supplemented with higher‐dose DHA: a randomised controlled trial. British Journal of Nutrition 2011;105(11):1635‐43. - PubMed
Collins 2015 {published data only}
-
- Collins CT, Gibson RA, Anderson PJ, McPhee AJ, Sullivan TR, Gould JF, et al. Neurodevelopmental outcomes at 7 years corrected age in preterm infants who were fed high‐dose docosahexaenoic acid to term equivalent: a follow up of a randomised controlled trial. BMJ Open 2015;5(3):e007314. - PMC - PubMed
Donzelli 1996 {published data only}
-
- Donzelli GP, Cafaggi L, Rapisardi G, Moroni M, Pratesi S. Longchain polyunsaturated fatty acids and early neural and visual development in preterm infants. Pediatric Research 1996;40:527.
Koletzko 2003 {published data only}
-
- Koletzko B, Sauerwald U, Keicher U, Saule H, Wawatschek S, Boehles H, et al. Fatty acid profiles, antioxidant status, and growth of preterm infants fed diets without or with long‐chain polyunsaturated fatty acids ‐ a randomized clinical trial. European Journal of Nutrition 2003;42(5):243‐53. - PubMed
Lim 2002 {published data only}
-
- Lim M, Antonson D, Clandinin M, vanAerde J, Green D, Stevens K, et al. Formulas with DHA and ARA for LBW infants are safe. Pediatric Research 2002;51:319A.
Makrides 2009 {published data only}
-
- Makrides M, Gibson RA, McPhee AJ, Collins CT, David PG, Doyle LW, et al. Neurodevelopmental outcomes of preterm infants fed high‐dose docosahexaenoic acid. Journal of the American Medical Association 2009;301(2):175‐82. - PubMed
Rodriguez 2003 {published data only}
-
- Rodriguez A, Raederstorff D, Sarda P, Lauret C, Mendy F, Descomps B. Preterm infant formula supplementation with alpha linoleic acid and docosahexaenoic acid. European Journal of Clinical Nutrition 2003;57(6):727‐34. - PubMed
Shah 2009 {published data only}
-
- Shah D. Is there any benefit of supplementing infant milk formulae with long chain polyunsaturated fatty acids. Indian Pediatrics 2009;46(9):783‐4. - PubMed
Smithers 2008a {published data only}
-
- Smithers LG, Gibson RA, McPhee A, Makrides M. Effect of two doses of docosahexaenoic acid (DHA) in the diet of preterm infants on infant fatty acid status: Results from the DINO trial. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2008;79(3‐5):141‐6. - PubMed
Smithers 2008b {published data only}
-
- Smithers LG, Gibson RA, McPhee A, Makrides M. Higher dose of docosaheaenoic acid in the neonatal period improves visual acuity of preterm infants: results of a randomized controlled trial. American Journal of Clinical Nutrition 2008;88(4):1049‐56. - PubMed
Smithers 2010 {published data only}
-
- Smithers LG, Collins CT, Simmonds LA, Gibson RA, McPhee AJ, Makrides M. Higher‐dose docosahexaenoic acid(DHA) does not influence language or behaviour: A follow up of the DINO (DHA for the improvement of neurodevelopemental outcome in preterm infants) trial. Journal of Paediatrics and Child Health. 2010; Vol. 46 (suppl.1):39.
van de Lagemaat 2011 {published data only}
-
- Lagemaat M, Rotteveel J, Muskiet FAJ, Schaafsma A, Lafeber HL. Post term dietary‐ induced changes in DHA and AA status relate to gains in weight, length, and head circumference in preterm infants. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2011;85(6):311‐6. - PubMed
Additional references
Anderson 1999
-
- Anderson JW, Johnstone BM, Remley DT. Breast‐feeding and cognitive development: a meta‐analysis. The American Journal of Clinical Nutrition 1999;70(4):525‐35. - PubMed
Beyerlein 2010
-
- Beyerlein A, Hadder‐Algra M, Kennedy K, Fewtrell M, Singhal A, Rosenfeld E, et al. Infant formula supplementation with long‐chain polyunsaturated fatty acids has no effect on bayley developmental scores at 18 months of age‐ IPD meta‐analysis of 4 large clinical trials. Journal of Pediatric Gastroenterology and Nutrition 2010;50(1):79‐84. - PubMed
Bjerve 1992
-
- Bjerve KS, Bredde OL, Bonaa K, Johnson H, Vatten L, Vik T. Clinical and epidemiological studies with alpha linolenic acid and longchain n‐3 fatty acids. In: Sinclair AJ, Gibson RA editor(s). Third International Conference on Essential Fatty Acids and Eicosanoids. Illinois: AOCS, March 1992.
Clandinin 1980
-
- Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid secretion rates in human brain: implications for fatty acid requirements. Early Human Development 1980;4(2):121‐9. - PubMed
Clark 1992
-
- Clark KJ, Makrides M, Neumann MA, Gibson RA. Determination of the optimal ratio of linoleic acid to alpha linolenic acid in infant formulas. Journal of Pediatrics 1992;120(4 Pt 2):S151‐8. - PubMed
Egger 1997
Fagan 1970
-
- Fagan JF 3rd. Memory in the infant. Journal of Experimental Child Psychology 1970;9(2):217‐26. - PubMed
Fagan 1983
-
- Fagan JF, Singer LT. Infant recognition memory as a measure of intelligence. In: Lipsitt LP editor(s). Advances in Infant Research. Vol. 2, Ablex, Norwood, 1983:31‐72.
GRADEpro [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Henriksen 2008
-
- Henriksen C, Haugholt K, Lindgren M, Aurvåg AK, Rønnestad A, Grønn M, et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics 2008;121(6):1137‐45. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jiao 2014
-
- Jiao J, Li Q, Chu J, Zeng W, Yang M, Zhu S. Effect of n‐3 PUFA supplementation on cognitive function throughout the life span from infancy to old age: a systematic review and meta‐analysis of randomized controlled trials. American Journal of Clinical Nutrition 2014;100(6):1422‐36. - PubMed
Kramer 2008
-
- Kramer MS, Aboud F, Mironova E, Vanilovich I, Platt RW, Matush L, et al. Breastfeeding and child cognitive development: new evidence from a large randomized trial. Archives of General Psychiatry 2008;65(5):578‐84. - PubMed
Lucas 1992
-
- Lucas A, Morley R, Cole TJ, Lister G, Leeson‐Payne C. Breastmilk and subsequent intelligence quotient in children born preterm. Lancet 1992;339(8788):261‐4. - PubMed
Makrides 1993
-
- Makrides M, Simmer K, Goggin M, Gibson RA. Erythrocyte docosahexaenoic acid correlates with the visual response of the healthy, term infant. Pediatric Research 1993;33(4 Pt 1):425‐7. - PubMed
Makrides 2015
-
- Makrides M, Kleinman RE. The long and short of it: Long‐chain fatty acids and long‐term outcomes for premature infants. Pediatrics 2015;135(6):1128‐9. - PubMed
Meldrum 2011
-
- Meldrum SJ, Smith MA, Prescott SL, Hird K, Simmer K. Achieving definitive results in long‐chain polyunsaturated fatty acid supplementation trials of term infants: factors for consideration. Nutrition Reviews 2011;69(4):205‐14. - PubMed
Morrow‐Tlucak 1988
-
- Morrow‐Tlucak M, Haude RH, Ernhart CB. Breastfeeding and cognitive development in the first two years of life. Social Science & Medicine 1988;26(6):635‐9. - PubMed
Neuringer 1986
Qawasmi 2012
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations.. Available from www.guidelinedevelopment.org/handbook Updated October 2013.
References to other published versions of this review
Schulzke 2011
Simmer 2000
Simmer 2004
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

