Keloid progression: a stiffness gap hypothesis
- PMID: 27995750
- PMCID: PMC7950128
- DOI: 10.1111/iwj.12693
Keloid progression: a stiffness gap hypothesis
Abstract
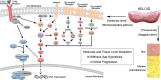
Keloids are fibroproliferative skin disorders characterised clinically by continuous horizontal progression and post-surgical recurrence and histologically by the accumulation of collagen and fibroblast ingredients. Till now, their aetiology remains clear, which may cover genetic, environmental and metabolic factors. Evidence in the involvement of local mechanics (e.g. predilection site and typical shape) and the progress in mechanobiology have incubated our stiffness gap hypotheses in illustrating the chronic but constant development in keloid. We put forward that the enlarged gap between extracellular matrix (ECM) stiffness and cellular stiffness potentiates keloid progression. Matrix stiffness itself provides organisational guidance cues to regulate the mechanosensitive resident cells (e.g. proliferation, migration and apoptosis). During this dynamic process, the ECM stiffness and cell stiffness are not well balanced, and the continuously enlarged stiffness gap between them potentiates keloid progression. The cushion factors, such as prestress for cell stiffness and topology for ECM stiffness, serve as compensations, the decompensation of which aggravates keloid development. It can well explain the typical shape of keloids, their progression in a horizontal but not vertical direction and the post-surgical recurrence, which were evidenced by our clinical cases. Such a stiffness gap hypothesis might be bridged to mechanotherapeutic approaches for keloid progression.
Keywords: Extracellular cell matrix stiffness; Keloid; Mechanobiology; Mechanoresponsiveness; Scar pathology.
© 2016 Medicalhelplines.com Inc and John Wiley & Sons Ltd.
Figures



References
-
- Marneros AG, Norris JE, Watanabe S, Reichenberger E, Olsen BR. Genome scans provide evidence for keloid susceptibility loci on chromosomes 2q23 and 7p11. J Invest Dermatol 2004;122:1126–32. - PubMed
-
- Ogawa R. Keloid and hypertrophic scarring may result from a mechanoreceptor or mechanosensitive nociceptor disorder. Med Hypotheses 2008;71:493–500. - PubMed
-
- Mowlem R. Hypertrophic scars. Br J Plast Surg 1951;4:113–20. - PubMed
-
- Fong EP, Bay BH. Keloids – the sebum hypothesis revisited. Med Hypotheses 2002;58:264–9. - PubMed
-
- Akaishi S, Ogawa R, Hyakusoku H. Keloid and hypertrophic scar: neurogenic inflammation hypotheses. Med Hypotheses 2008;71:32–8. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

