Regulatory T cells in cancer immunotherapy
- PMID: 27995907
- PMCID: PMC5223231
- DOI: 10.1038/cr.2016.151
Regulatory T cells in cancer immunotherapy
Abstract
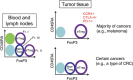
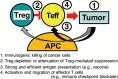
FOXP3-expressing regulatory T (Treg) cells, which suppress aberrant immune response against self-antigens, also suppress anti-tumor immune response. Infiltration of a large number of Treg cells into tumor tissues is often associated with poor prognosis. There is accumulating evidence that the removal of Treg cells is able to evoke and enhance anti-tumor immune response. However, systemic depletion of Treg cells may concurrently elicit deleterious autoimmunity. One strategy for evoking effective tumor immunity without autoimmunity is to specifically target terminally differentiated effector Treg cells rather than all FOXP3+ T cells, because effector Treg cells are the predominant cell type in tumor tissues. Various cell surface molecules, including chemokine receptors such as CCR4, that are specifically expressed by effector Treg cells can be the candidates for depleting effector Treg cells by specific cell-depleting monoclonal antibodies. In addition, other immunological characteristics of effector Treg cells, such as their high expression of CTLA-4, active proliferation, and apoptosis-prone tendency, can be exploited to control specifically their functions. For example, anti-CTLA-4 antibody may kill effector Treg cells or attenuate their suppressive activity. It is hoped that combination of Treg-cell targeting (e.g., by reducing Treg cells or attenuating their suppressive activity in tumor tissues) with the activation of tumor-specific effector T cells (e.g., by cancer vaccine or immune checkpoint blockade) will make the current cancer immunotherapy more effective.
Figures

References
-
- Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J Immunol 2004; 172:5967–5972. - PubMed
-
- Nishikawa H, Jäger E, Ritter G, Old LJ, Gnjatic S. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood 2005; 106:1008–1011. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

