Engineering the surface properties of a human monoclonal antibody prevents self-association and rapid clearance in vivo
- PMID: 27995962
- PMCID: PMC5171805
- DOI: 10.1038/srep38644
Engineering the surface properties of a human monoclonal antibody prevents self-association and rapid clearance in vivo
Abstract
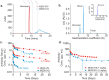
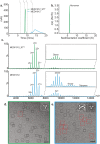
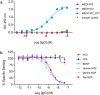
Uncontrolled self-association is a major challenge in the exploitation of proteins as therapeutics. Here we describe the development of a structural proteomics approach to identify the amino acids responsible for aberrant self-association of monoclonal antibodies and the design of a variant with reduced aggregation and increased serum persistence in vivo. We show that the human monoclonal antibody, MEDI1912, selected against nerve growth factor binds with picomolar affinity, but undergoes reversible self-association and has a poor pharmacokinetic profile in both rat and cynomolgus monkeys. Using hydrogen/deuterium exchange and cross-linking-mass spectrometry we map the residues responsible for self-association of MEDI1912 and show that disruption of the self-interaction interface by three mutations enhances its biophysical properties and serum persistence, whilst maintaining high affinity and potency. Immunohistochemistry suggests that this is achieved via reduction of non-specific tissue binding. The strategy developed represents a powerful and generic approach to improve the properties of therapeutic proteins.
Conflict of interest statement
The MedImmune authors are employees of the AstraZeneca Group and have stock/stock options in AstraZeneca. J.J.P. acknowledges financial support from MedImmune via The Beacon Project.
Figures



References
-
- Vugmeyster Y. et al.. Complex pharmacokinetics of a humanized antibody against human amyloid beta peptide, anti-abeta Ab2, in nonclinical species. Pharm. Res. 28, 1696–1706 (2011). - PubMed
-
- Wu H. et al.. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J. Mol. Biol. 368, 652–665 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/E012558/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J011819/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 322408/ERC_/European Research Council/International
- BB/M012573/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 090932/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

