Covalent Modifiers: A Chemical Perspective on the Reactivity of α,β-Unsaturated Carbonyls with Thiols via Hetero-Michael Addition Reactions
- PMID: 27996267
- PMCID: PMC5308545
- DOI: 10.1021/acs.jmedchem.6b00788
Covalent Modifiers: A Chemical Perspective on the Reactivity of α,β-Unsaturated Carbonyls with Thiols via Hetero-Michael Addition Reactions
Abstract
Although Michael acceptors display a potent and broad spectrum of bioactivity, they have largely been ignored in drug discovery because of their presumed indiscriminate reactivity. As such, a dearth of information exists relevant to the thiol reactivity of natural products and their analogues possessing this moiety. In the midst of recently approved acrylamide-containing drugs, it is clear that a good understanding of the hetero-Michael addition reaction and the relative reactivities of biological thiols with Michael acceptors under physiological conditions is needed for the design and use of these compounds as biological tools and potential therapeutics. This Perspective provides information that will contribute to this understanding, such as kinetics of thiol addition reactions, bioactivities, as well as steric and electronic factors that influence the electrophilicity and reversibility of Michael acceptors. This Perspective is focused on α,β-unsaturated carbonyls given their preponderance in bioactive natural products.
Figures
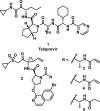
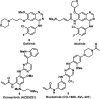
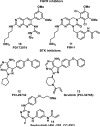
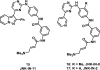
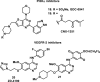
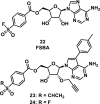
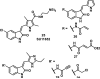
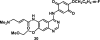
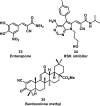
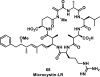
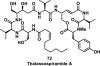
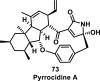
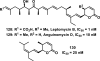
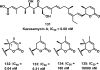
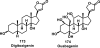
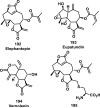
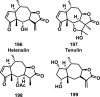
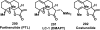
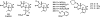
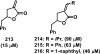

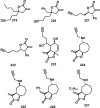
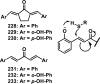
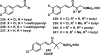
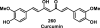
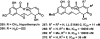
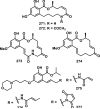
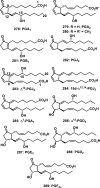
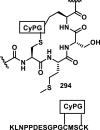
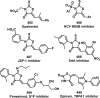
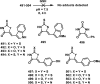
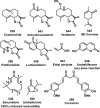
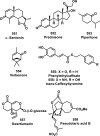
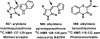

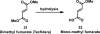
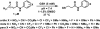
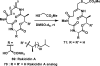

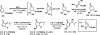
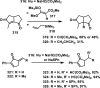
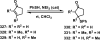
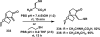
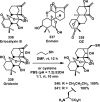
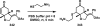
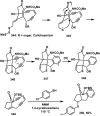
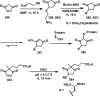
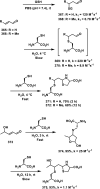
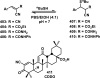
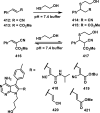

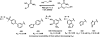
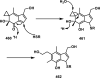
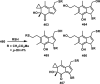
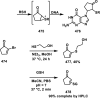
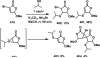
References
-
- Schöwobel JAH, Koleva YK, Enoch SJ, Bajot F, Hewitt M, Madden JC, Roberts DW, Schultz TW, Cronin MTD. Measurement and Estimation of Electrophilic Reactivity for Predictive Toxicology. Chem. Rev. 2011;111:2562–2596. - PubMed
-
- Singh J, Petter RC, Baillie TA, Whitty A. The Resurgence of Covalent Drugs. Nat. Rev. Drug Disc. 2011;10:307–317. - PubMed
-
- Parsons ZD, Gates KS. Redox Regulation of Protein Tyrosine Phosphatases: Methods for Kinetic Analysis of Covalent Enzyme Inactivation. In: Cadenas E, Packer L, editors. Methods in Enzymology Volume 528: Hydrogen Peroxide and Cell Signaling Part C. Vol. 528. Elsevier Science Publishing Co Inc; San Diego: 2013. pp. 130–154. - PubMed
- Kitz R, Wilson IB. Esters of Methanesulfonic Acid as Irreversible Inhibitors of Acetylcholinesterase. J. Biol. Chem. 1962;237:3245–3249. - PubMed
- Nagahara N, Sawada N, Nakagawa T. Affinity Labeling of a Catalytic Site, Cysteine 247, in Rat Mercaptopyruvate Sulfurtransfera se by Chloropyruvate as an Analog of a Substrate. Biochimie. 2004;86:723–729. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

