Differential Sensitivity to Ethanol-Induced Circadian Rhythm Disruption in Adolescent and Adult Mice
- PMID: 27997028
- PMCID: PMC5205578
- DOI: 10.1111/acer.13275
Differential Sensitivity to Ethanol-Induced Circadian Rhythm Disruption in Adolescent and Adult Mice
Abstract
Background: Growing evidence supports a central role for the circadian system in alcohol use disorders, but few studies have examined this relationship during adolescence. In mammals, circadian rhythms are regulated by the suprachiasmatic nucleus, a biological clock whose timing is synchronized (reset) to the environment primarily by light (photic) input. Alcohol (ethanol [EtOH]) disrupts circadian timing in part by attenuating photic phase-resetting responses in adult rodents. However, circadian rhythms change throughout life and it is not yet known whether EtOH has similar effects on circadian regulation during adolescence.
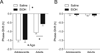
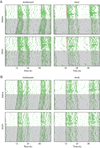
Methods: General circadian locomotor activity was monitored in male C57BL6/J mice beginning in adolescence (P27) or adulthood (P61) in a 12-hour light, 12-hour dark photocycle for ~2 weeks to establish baseline circadian activity measures. On the day of the experiment, mice received an acute injection of EtOH (1.5 g/kg, i.p.) or equal volume saline 15 minutes prior to a 30-minute light pulse at Zeitgeber Time 14 (2 hours into the dark phase) and then were released into constant darkness (DD) for ~2 weeks to assess phase-resetting responses. Control mice of each age-group received injections but no light pulse prior to DD.
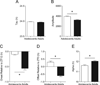
Results: While adults showed the expected decrease in photic phase-delays induced by acute EtOH, this effect was absent in adolescent mice. Adolescents also showed baseline differences in circadian rhythmicity compared to adults, including advanced photocycle entrainment, larger photic phase-delays, a shorter free-running (endogenous) circadian period, and greater circadian rhythm amplitude.
Conclusions: Collectively, our results indicate that adolescent mice are less sensitive to the effect of EtOH on circadian photic phase-resetting and that their daily activity rhythms are markedly different than those of adults.
Keywords: Adolescent; Alcohol; Circadian; Photic Phase-Resetting.
Copyright © 2016 by the Research Society on Alcoholism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Abe H, Rusak B, Robertson HA. Photic induction of Fos protein in the suprachiasmatic nucleus is inhibited by the NMDA receptor antagonist MK-801. Neurosci Lett. 1991;127:9–12. - PubMed
-
- Albert N, da Silva C, Diez-Noguera A, Cambras T. Different adaptation of the motor activity rhythm to chronic phase shifts between adolescent and adult rats. Behav Brain Res. 2013;252:347–355. - PubMed
-
- Aschoff J. Response curves in circadian periodicity. In: Aschoff J, editor. Circadian Clocks. Amsterdam: North-Holland; 1965. pp. 95–111.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

