Pathological correlations of [F-18]-AV-1451 imaging in non-alzheimer tauopathies
- PMID: 27997036
- PMCID: PMC5319193
- DOI: 10.1002/ana.24844
Pathological correlations of [F-18]-AV-1451 imaging in non-alzheimer tauopathies
Abstract
Objective: Recent studies have shown that positron emission tomography (PET) tracer AV-1451 exhibits high binding affinity for paired helical filament (PHF)-tau pathology in Alzheimer's brains. However, the ability of this ligand to bind to tau lesions in other tauopathies remains controversial. Our goal was to examine the correlation of in vivo and postmortem AV-1451 binding patterns in three autopsy-confirmed non-Alzheimer tauopathy cases.
Methods: We quantified in vivo retention of [F-18]-AV-1451 and performed autoradiography, [H-3]-AV-1451 binding assays, and quantitative tau measurements in postmortem brain samples from two progressive supranuclear palsy (PSP) cases and a MAPT P301L mutation carrier. They all underwent [F-18]-AV-1451 PET imaging before death.
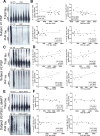
Results: The three subjects exhibited [F-18]-AV-1451 in vivo retention predominantly in basal ganglia and midbrain. Neuropathological examination confirmed the PSP diagnosis in the first two subjects; the MAPT P301L mutation carrier had an atypical tauopathy characterized by grain-like tau-containing neurites in gray and white matter with heaviest burden in basal ganglia. In all three cases, autoradiography failed to show detectable [F-18]-AV-1451 binding in multiple brain regions examined, with the exception of entorhinal cortex (reflecting incidental age-related neurofibrillary tangles) and neuromelanin-containing neurons in the substantia nigra (off-target binding). The lack of a consistent significant correlation between in vivo [F-18]-AV-1541 retention and postmortem in vitro binding and tau measures in these cases suggests that this ligand has low affinity for tau lesions primarily made of straight tau filaments.
Interpretation: AV-1451 may have limited utility for in vivo selective and reliable detection of tau aggregates in these non-Alzheimer tauopathies. ANN NEUROL 2017;81:117-128.
© 2016 American Neurological Association.
Conflict of interest statement
Potential Conflict of Interest:
Bradley T. Hyman reports personal fees from Lilly, the company that owns [F-18]-AV-1451, outside the submitted work.
Keith A. Johnson reports personal fees from Lilly/Avid, the company that owns [F-18]-AV-1451, outside the submitted work.
All other authors have nothing to disclose.
Figures




References
-
- Shah M, Catafau AM. Molecular Imaging Insights into Neurodegeneration: Focus on Tau PET Radiotracers. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2014 Jun;55(6):871–4. - PubMed
-
- Villemagne VL, Fodero-Tavoletti MT, Masters CL, et al. Tau imaging: early progress and future directions. The Lancet Neurology. 2015 Jan;14(1):114–24. - PubMed
-
- Xia CF, Arteaga J, Chen G, et al. [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2013 Nov;9(6):666–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

