Descending projections from the basal forebrain to the orexin neurons in mice
- PMID: 27997037
- PMCID: PMC5806522
- DOI: 10.1002/cne.24158
Descending projections from the basal forebrain to the orexin neurons in mice
Abstract
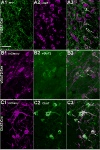
The orexin (hypocretin) neurons play an essential role in promoting arousal, and loss of the orexin neurons results in narcolepsy, a condition characterized by chronic sleepiness and cataplexy. The orexin neurons excite wake-promoting neurons in the basal forebrain (BF), and a reciprocal projection from the BF back to the orexin neurons may help promote arousal and motivation. The BF contains at least three different cell types (cholinergic, glutamatergic, and γ-aminobutyric acid (GABA)ergic neurons) across its different regions (medial septum, diagonal band, magnocellular preoptic area, and substantia innominata). Given the neurochemical and anatomical heterogeneity of the BF, we mapped the pattern of BF projections to the orexin neurons across multiple BF regions and neuronal types. We performed conditional anterograde tracing using mice that express Cre recombinase only in neurons producing acetylcholine, glutamate, or GABA. We found that the orexin neurons are heavily apposed by axon terminals of glutamatergic and GABAergic neurons of the substantia innominata (SI) and magnocellular preoptic area, but there was no innervation by the cholinergic neurons. Channelrhodopsin-assisted circuit mapping (CRACM) demonstrated that glutamatergic SI neurons frequently form functional synapses with the orexin neurons, but, surprisingly, functional synapses from SI GABAergic neurons were rare. Considering their strong reciprocal connections, BF and orexin neurons likely work in concert to promote arousal, motivation, and other behaviors. J. Comp. Neurol. 525:1668-1684, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: CRACM; RRID: AB_10013483; RRID: AB_11180610; RRID: AB_262156; RRID: AB_653610; anterograde; hypocretin; lateral hypothalamus; magnocellular preoptic; mice; substantia innominata.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
Figures






References
-
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. - PubMed
-
- Bayer L, Eggermann E, Serafin M, Grivel J, Machard D, Muhlethaler M, Jones BE. Opposite effects of noradrenaline and acetylcholine upon hypocretin/orexin versus melanin concentrating hormone neurons in rat hypothalamic slices. Neuroscience. 2005;130(4):807–811. - PubMed
-
- Blanco-Centurion CA, Shiromani A, Winston E, Shiromani PJ. Effects of hypocretin-1 in 192-IgG-saporin-lesioned rats. Eur J Neurosci. 2006;24(7):2084–2088. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

