Comprehensive Insights into the Multi-Antioxidative Mechanisms of Melanin Nanoparticles and Their Application To Protect Brain from Injury in Ischemic Stroke
- PMID: 27997170
- PMCID: PMC5752099
- DOI: 10.1021/jacs.6b11013
Comprehensive Insights into the Multi-Antioxidative Mechanisms of Melanin Nanoparticles and Their Application To Protect Brain from Injury in Ischemic Stroke
Abstract
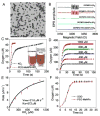
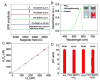
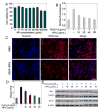
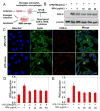
Nanotechnology-mediated antioxidative therapy is emerging as a novel strategy for treating a myriad of important diseases through scavenging excessive reactive oxygen and nitrogen species (RONS), a mechanism critical in disease development and progression. However, similar to antioxidative enzymes, currently studied nanoantioxidants have demonstrated scavenging activity to specific RONS, and sufficient antioxidative effects against multiple RONS generated in diseases remain elusive. Here we propose to develop bioinspired melanin nanoparticles (MeNPs) for more potent and safer antioxidative therapy. While melanin is known to function as a potential radical scavenger, its antioxidative mechanisms are far from clear, and its applications for the treatment of RONS-associated diseases have yet to be well-explored. In this study, we provide for the first time exhaustive characterization of the activities of MeNPs against multiple RONS including O2•-, H2O2, •OH, •NO, and ONOO-, the main toxic RONS generated in diseases. The potential of MeNPs for antioxidative therapy has also been evaluated in vitro and in a rat model of ischemic stroke. In addition to the broad defense against these RONS, MeNPs can also attenuate the RONS-triggered inflammatory responses through suppressing the expression of inflammatory mediators and cytokines. In vivo results further demonstrate that these unique multi-antioxidative, anti-inflammatory, and biocompatible features of MeNPs contribute to their effective protection of ischemic brains with negligible side effects.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Hollow Prussian Blue Nanozymes Drive Neuroprotection against Ischemic Stroke via Attenuating Oxidative Stress, Counteracting Inflammation, and Suppressing Cell Apoptosis.Nano Lett. 2019 May 8;19(5):2812-2823. doi: 10.1021/acs.nanolett.8b04729. Epub 2019 Apr 4. Nano Lett. 2019. PMID: 30908916
-
Site-Specific Biomimicry of Antioxidative Melanin Formation and Its Application for Acute Liver Injury Therapy and Imaging.Adv Mater. 2021 Aug;33(34):e2102391. doi: 10.1002/adma.202102391. Epub 2021 Jul 18. Adv Mater. 2021. PMID: 34278624
-
Hydroxyl Radical-Suppressing Mechanism and Efficiency of Melanin-Mimetic Nanoparticles.Int J Mol Sci. 2018 Aug 7;19(8):2309. doi: 10.3390/ijms19082309. Int J Mol Sci. 2018. PMID: 30087240 Free PMC article.
-
Melanin nanoparticles as a promising tool for biomedical applications - a review.Acta Biomater. 2020 Mar 15;105:26-43. doi: 10.1016/j.actbio.2020.01.044. Epub 2020 Feb 1. Acta Biomater. 2020. PMID: 32014585 Review.
-
Nanomedicine and nanobiotechnology applications of magnetoelectric nanoparticles.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023 Mar;15(2):e1849. doi: 10.1002/wnan.1849. Epub 2022 Sep 3. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023. PMID: 36056752 Review.
Cited by
-
Reprogramming the myocardial infarction microenvironment with melanin-based composite nanomedicines in mice.Nat Commun. 2024 Aug 6;15(1):6651. doi: 10.1038/s41467-024-50854-4. Nat Commun. 2024. PMID: 39103330 Free PMC article.
-
Nanotechnology approaches to drug delivery for the treatment of ischemic stroke.Bioact Mater. 2024 Sep 23;43:145-161. doi: 10.1016/j.bioactmat.2024.09.016. eCollection 2025 Jan. Bioact Mater. 2024. PMID: 39386225 Free PMC article. Review.
-
Melanin and Melanin-Like Hybrid Materials in Regenerative Medicine.Nanomaterials (Basel). 2020 Aug 3;10(8):1518. doi: 10.3390/nano10081518. Nanomaterials (Basel). 2020. PMID: 32756369 Free PMC article. Review.
-
Scavenging of reactive oxygen and nitrogen species with nanomaterials.Nano Res. 2018 Oct;11(10):4955-4984. doi: 10.1007/s12274-018-2092-y. Epub 2018 May 26. Nano Res. 2018. PMID: 30450165 Free PMC article.
-
Isolation and Characterization of Allomelanin from Pathogenic Black Knot Fungus-a Sustainable Source of Melanin.ACS Omega. 2021 Dec 14;6(51):35514-35522. doi: 10.1021/acsomega.1c05030. eCollection 2021 Dec 28. ACS Omega. 2021. PMID: 34984283 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

