Phosphatidylserine Ameliorates Neurodegenerative Symptoms and Enhances Axonal Transport in a Mouse Model of Familial Dysautonomia
- PMID: 27997532
- PMCID: PMC5172536
- DOI: 10.1371/journal.pgen.1006486
Phosphatidylserine Ameliorates Neurodegenerative Symptoms and Enhances Axonal Transport in a Mouse Model of Familial Dysautonomia
Abstract
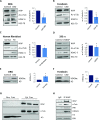
Familial Dysautonomia (FD) is a neurodegenerative disease in which aberrant tissue-specific splicing of IKBKAP exon 20 leads to reduction of IKAP protein levels in neuronal tissues. Here we generated a conditional knockout (CKO) mouse in which exon 20 of IKBKAP is deleted in the nervous system. The CKO FD mice exhibit developmental delays, sensory abnormalities, and less organized dorsal root ganglia (DRGs) with attenuated axons compared to wild-type mice. Furthermore, the CKO FD DRGs show elevated HDAC6 levels, reduced acetylated α-tubulin, unstable microtubules, and impairment of axonal retrograde transport of nerve growth factor (NGF). These abnormalities in DRG properties underlie neuronal degeneration and FD symptoms. Phosphatidylserine treatment decreased HDAC6 levels and thus increased acetylation of α-tubulin. Further PS treatment resulted in recovery of axonal outgrowth and enhanced retrograde axonal transport by decreasing histone deacetylase 6 (HDAC6) levels and thus increasing acetylation of α-tubulin levels. Thus, we have identified the molecular pathway that leads to neurodegeneration in FD and have demonstrated that phosphatidylserine treatment has the potential to slow progression of neurodegeneration.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Animal and cellular models of familial dysautonomia.Clin Auton Res. 2017 Aug;27(4):235-243. doi: 10.1007/s10286-017-0438-2. Epub 2017 Jun 30. Clin Auton Res. 2017. PMID: 28667575 Free PMC article. Review.
-
Phosphatidylserine increases IKBKAP levels in a humanized knock-in IKBKAP mouse model.Hum Mol Genet. 2013 Jul 15;22(14):2785-94. doi: 10.1093/hmg/ddt126. Epub 2013 Mar 20. Hum Mol Genet. 2013. PMID: 23515154
-
Involvement of IKAP in peripheral target innervation and in specific JNK and NGF signaling in developing PNS neurons.PLoS One. 2014 Nov 19;9(11):e113428. doi: 10.1371/journal.pone.0113428. eCollection 2014. PLoS One. 2014. PMID: 25409162 Free PMC article.
-
Sensory and autonomic deficits in a new humanized mouse model of familial dysautonomia.Hum Mol Genet. 2016 Mar 15;25(6):1116-28. doi: 10.1093/hmg/ddv634. Epub 2016 Jan 13. Hum Mol Genet. 2016. PMID: 26769677 Free PMC article.
-
Phosphatidylserine improves axonal transport by inhibition of HDAC and has potential in treatment of neurodegenerative diseases.Neural Regen Res. 2017 Apr;12(4):534-537. doi: 10.4103/1673-5374.205082. Neural Regen Res. 2017. PMID: 28553323 Free PMC article. Review.
Cited by
-
Emerging roles of histone modifications and HDACs in RNA splicing.Nucleic Acids Res. 2019 Jun 4;47(10):4911-4926. doi: 10.1093/nar/gkz292. Nucleic Acids Res. 2019. PMID: 31162605 Free PMC article. Review.
-
Elp1 is required for development of visceral sensory peripheral and central circuitry.Dis Model Mech. 2022 May 1;15(5):dmm049274. doi: 10.1242/dmm.049274. Epub 2022 Jun 1. Dis Model Mech. 2022. PMID: 35481599 Free PMC article.
-
ELP1 Splicing Correction Reverses Proprioceptive Sensory Loss in Familial Dysautonomia.Am J Hum Genet. 2019 Apr 4;104(4):638-650. doi: 10.1016/j.ajhg.2019.02.009. Epub 2019 Mar 21. Am J Hum Genet. 2019. PMID: 30905397 Free PMC article.
-
Identification of key molecular biomarkers involved in reactive and neurodegenerative processes present in inherited congenital hydrocephalus.Fluids Barriers CNS. 2021 Jul 2;18(1):30. doi: 10.1186/s12987-021-00263-2. Fluids Barriers CNS. 2021. PMID: 34215285 Free PMC article.
-
Animal and cellular models of familial dysautonomia.Clin Auton Res. 2017 Aug;27(4):235-243. doi: 10.1007/s10286-017-0438-2. Epub 2017 Jun 30. Clin Auton Res. 2017. PMID: 28667575 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

