Natalizumab-Related Progressive Multifocal Leukoencephalopathy in Multiple Sclerosis: Findings from an Italian Independent Registry
- PMID: 27997580
- PMCID: PMC5172579
- DOI: 10.1371/journal.pone.0168376
Natalizumab-Related Progressive Multifocal Leukoencephalopathy in Multiple Sclerosis: Findings from an Italian Independent Registry
Abstract
Background: The monoclonal antibody natalizumab (NTZ) is a highly effective treatment for patients with multiple sclerosis (MS). However, this drug is associated with increased risk of developing Progressive Multifocal Leukoencephalopathy (PML), an opportunistic infection of central nervous system (CNS) caused by the John Cunningham polyomavirus (JCV).
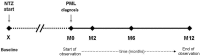
Objective: To describe the 12-month clinical course of 39 patients with MS (28 women, 11 men) who developed NTZ-related PML after a mean exposure of 39 infusions.
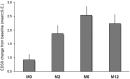
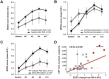
Methods: An Italian independent collaborative repository initiative collected and analyzed socio-demographic, clinical, magnetic resonance imaging (MRI) data and number of JCV-DNA copies detected on cerebrospinal fluid (CSF) samples of patients diagnosed as affected by NTZ-related PML. The evolution of disability, measured by the Expanded Disability Status Scale, was assessed at NTZ start, at PML diagnosis and after 2, 6 and 12 months from PML diagnosis. The effect of clinical and paraclinical characteristics at PML diagnosis on the final outcome was also investigated.
Results: Ten patients (25.6%) were diagnosed before 24 NTZ infusions. In six cases (15.4%) the PML suspect was made on the basis of highly suggestive MRI findings in absence of any detectable change of clinical conditions (asymptomatic PML). In patients with symptomatic PML, the diagnosis was quicker for those who presented with cognitive symptoms (n = 12) rather than for those with other neurological pictures (n = 21) (p = 0.003). Three patients (7.7%) died during the 12-month observation period, resulting in a survival rate of 92.3%. Asymptomatic PML, more localized brain involvement and gadolinium-enhancement detected at MRI, as well as lower viral load were associated with a better disability outcome (p-values<0.01).
Conclusion: Our findings support that early PML diagnosis, limited CNS involvement and initial signs of immune restoration are associated with a better outcome and higher survival rate, and confirm the utility of MRI as a surveillance tool for NTZ-treated patients.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: Dr. Prosperini declares received consulting fees from Biogen and Novartis; speaker honoraria from Biogen, Genzyme, Novartis and Teva; travel grants from Biogen, Genzyme, Novartis and Teva; research grants from the Italian MS Society (Associazione Italiana Sclerosi Multipla) and Genzyme. Dr. Prosperini also acts as member of steering committee AIFA (Agenzia Italiana del Farmaco) on natalizumab. Dr. De Rossi received speaker honoraria from Biogen and Teva and travel grants from Biogen, Teva and Merk Serono. Dr. Scarpazza has nothing to disclose. Dr. Moiola received honoraria for speaking or for advisory board from Sanofi-Genzyme, Biogen, Novartis and Teva. Dr. Cosottini received speaker honoraria from Biogen. Dr. Gerevini received speaker honoraria from Biogen. Dr. Capra received consulting fees from Novartis and Biogen, and lecture fees and/or travel grants from Novartis, Biogen, Genzyme and Sanofi-Aventis.
Figures
References
-
- Ferenczy MW, Marshall LJ, Nelson CD, Atwood WJ, Nath A, Khalili K, et al. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev 2012; 25(3): 471–506. 10.1128/CMR.05031-11 - DOI - PMC - PubMed
-
- Cinque P, Koralnik IJ, Clifford DB. The evolving face of human immunodeficiency virus-related progressive multifocal leukoencephalopathy: defining a consensus terminology. J Neurovirol. 2003; 9 Suppl 1: 88–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

