Ganglioside Profiling of the Human Retina: Comparison with Other Ocular Structures, Brain and Plasma Reveals Tissue Specificities
- PMID: 27997589
- PMCID: PMC5173345
- DOI: 10.1371/journal.pone.0168794
Ganglioside Profiling of the Human Retina: Comparison with Other Ocular Structures, Brain and Plasma Reveals Tissue Specificities
Abstract
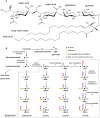
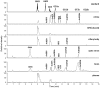
Gangliosides make a wide family of glycosphingolipids, highly heterogeneous in both the ceramide moiety and the oligosaccharide chain. While ubiquitously expressed in mammalian tissues, they are particularly abundant in the brain and the peripheral nervous system. Gangliosides are known to play a crucial role in the development, maintenance and functional integrity of the nervous system. However, the expression and roles of gangliosides in the retina, although often considered as a window on the brain, has been far less studied. We performed an in-depth analysis of gangliosides of the human retina, especially using powerful LC/MS methods. We compared the pattern of ganglioside classes and ceramide molecular species of this tissue with other ocular structures and with brain and plasma in elderly human individuals. About a hundred of ganglioside molecular species among 15 distinct classes were detected illustrating the huge structural diversity of these compounds. The retina exhibited a very diverse ganglioside profile and shared several common features with the brain (prominence of tetraosylgangliosides, abundance of d20:1 long chain base and 18:0 fatty acid…). However, the retina stood out with the specific expression of GD3, GT3 and AcGT3, which further presented a peculiar molecular species distribution. The unique ganglioside pattern we observed in the human retina suggests that these ganglioside species play a specific role in the structure and function of this tissue. This lipidomic study, by highlighting retina specific ganglioside species, opens up novel research directions for a better understanding of the biological role of gangliosides in the retina.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Svennerholm L. Ganglioside designation. Adv Exp Med Biol. 1980;125:11 - PubMed
-
- Yu RK, Yanagisawa M., Ariga T.. Glycosphingolipid Structures In: Kamerling JP, editor. Comprehensive Glycoscience. 1: Elsevier; 2007. p. 73–120.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

