Glucose Transporter 1-Dependent Glycolysis Is Increased during Aging-Related Lung Fibrosis, and Phloretin Inhibits Lung Fibrosis
- PMID: 27997810
- PMCID: PMC5449513
- DOI: 10.1165/rcmb.2016-0225OC
Glucose Transporter 1-Dependent Glycolysis Is Increased during Aging-Related Lung Fibrosis, and Phloretin Inhibits Lung Fibrosis
Abstract
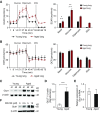
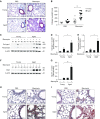
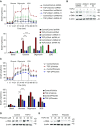
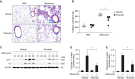
Aging is associated with metabolic diseases such as type 2 diabetes mellitus, cardiovascular disease, cancer, and neurodegeneration. Aging contributes to common processes including metabolic dysfunction, DNA damage, and reactive oxygen species generation. Although glycolysis has been linked to cell growth and proliferation, the mechanisms by which the activation of glycolysis by aging regulates fibrogenesis in the lung remain unclear. The objective of this study was to determine if glucose transporter 1 (GLUT1)-induced glycolysis regulates age-dependent fibrogenesis of the lung. Mouse and human lung tissues were analyzed for GLUT1 and glycolytic markers using immunoblotting. Glycolytic function was measured using a Seahorse apparatus. To study the effect of GLUT1, genetic inhibition of GLUT1 was performed by short hairpin RNA transduction, and phloretin was used for pharmacologic inhibition of GLUT1. GLUT1-dependent glycolysis is activated in aged lung. Genetic and pharmacologic inhibition of GLUT1 suppressed the protein expression of α-smooth muscle actin, a key cytoskeletal component of activated fibroblasts, in mouse primary lung fibroblast cells. Moreover, we demonstrated that the activation of AMP-activated protein kinase, which is regulated by GLUT1-dependent glycolysis, represents a critical metabolic pathway for fibroblast activation. Furthermore, we demonstrated that phloretin, a potent inhibitor of GLUT1, significantly inhibited bleomycin-induced lung fibrosis in vivo. These results suggest that GLUT1-dependent glycolysis regulates fibrogenesis in aged lung and that inhibition of GLUT1 provides a potential target of therapy of age-related lung fibrosis.
Keywords: bleomycin; glucose metabolism; idiopathic pulmonary fibrosis.
Figures



References
-
- Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. - PubMed
-
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

