Actin-based motility and cell-to-cell spread of bacterial pathogens
- PMID: 27997855
- PMCID: PMC5474209
- DOI: 10.1016/j.mib.2016.11.007
Actin-based motility and cell-to-cell spread of bacterial pathogens
Abstract
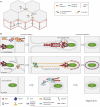
Subversion of the host actin cytoskeleton is a critical virulence mechanism used by a variety of intracellular bacterial pathogens during their infectious life cycles. These pathogens manipulate host actin to promote actin-based motility and coordinate motility with cell-to-cell spread. Growing evidence suggests that the tactics employed by pathogens are surprisingly diverse. Here, we review recent advances suggesting that bacterial surface proteins exhibit divergent biochemical mechanisms of actin polymerization and recruit distinct host protein networks to drive motility, and that bacteria deploy secreted effector proteins that alter host cell mechanotransduction pathways to enable spread. Further investigation into the divergent strategies used by bacterial pathogens to mobilize actin will reveal new insights into pathogenesis and cytoskeleton regulation.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

