Melanocortin receptor agonists MCR1-5 protect photoreceptors from high-glucose damage and restore antioxidant enzymes in primary retinal cell culture
- PMID: 27998021
- PMCID: PMC5387132
- DOI: 10.1111/jcmm.13036
Melanocortin receptor agonists MCR1-5 protect photoreceptors from high-glucose damage and restore antioxidant enzymes in primary retinal cell culture
Abstract
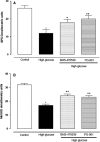
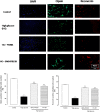
Retinal photoreceptors are particularly vulnerable to local high-glucose concentrations. Oxidative stress is a risk factor for diabetic retinopathy development. Melanocortin receptors represent a family of G-protein-coupled receptors classified in five subtypes and are expressed in retina. Our previous data indicate that subtypes 1 and 5 receptor agonists exert a protective role on experimental diabetic retinopathy. This study focuses on their role in primary retinal cell cultures in high-glucose concentrations. After eye enucleation from wild-type male C57BL/6 mice, retinal cells were isolated, plated in high-glucose concentration and treated with melanocortin receptors 1 and 5 agonists and antagonists. Immunocytochemical and biochemical analysis showed that treatment with melanocortin receptors 1 and 5 agonists reduced anti-inflammatory cytokines and chemokines and enhanced manganese superoxide dismutase and glutathione peroxidase levels, preserving photoreceptor integrity. According with these evidences, we propose a major role of melanocortin receptors 1 and 5 on primary retinal cell response against high glucose or oxidative insults.
Keywords: hyperglycaemia; melanocortin receptor agonists; oxidative stress; photoreceptors; primary retinal cell cultures.
© 2016 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures


Similar articles
-
Hesperetin rescues retinal oxidative stress, neuroinflammation and apoptosis in diabetic rats.Microvasc Res. 2013 May;87:65-74. doi: 10.1016/j.mvr.2013.01.002. Epub 2013 Jan 31. Microvasc Res. 2013. PMID: 23376836
-
Effects of antioxidant treatment on normal and diabetic rat retinal enzyme activities.J Ocul Pharmacol Ther. 2005 Feb;21(1):28-35. doi: 10.1089/jop.2005.21.28. J Ocul Pharmacol Ther. 2005. PMID: 15718825
-
Altered mRNA levels of antioxidant enzymes in pre-apoptotic pericytes from human diabetic retinas.Cell Mol Biol (Noisy-le-grand). 1999 Feb;45(1):59-66. Cell Mol Biol (Noisy-le-grand). 1999. PMID: 10099840
-
Effects of L-carnitine on high glucose-induced oxidative stress in retinal ganglion cells.Pharmacology. 2014;94(3-4):123-30. doi: 10.1159/000363062. Epub 2014 Sep 17. Pharmacology. 2014. PMID: 25247444
-
Glutathione peroxidase contributes with heme oxygenase-1 to redox balance in mouse brain during the course of cerebral malaria.Biochim Biophys Acta. 2013 Dec;1832(12):2009-18. doi: 10.1016/j.bbadis.2013.07.010. Epub 2013 Jul 18. Biochim Biophys Acta. 2013. PMID: 23872112
Cited by
-
Therapeutic Effects of Stimulating the Melanocortin Pathway in Regulating Ocular Inflammation and Cell Death.Biomolecules. 2024 Jan 31;14(2):169. doi: 10.3390/biom14020169. Biomolecules. 2024. PMID: 38397406 Free PMC article. Review.
-
The Melanocortin System in Inflammatory Bowel Diseases: Insights into Its Mechanisms and Therapeutic Potentials.Cells. 2023 Jul 19;12(14):1889. doi: 10.3390/cells12141889. Cells. 2023. PMID: 37508552 Free PMC article. Review.
-
ARPE-19-derived VEGF-containing exosomes promote neovascularization in HUVEC: the role of the melanocortin receptor 5.Cell Cycle. 2019 Feb;18(4):413-424. doi: 10.1080/15384101.2019.1568745. Epub 2019 Feb 9. Cell Cycle. 2019. PMID: 30739530 Free PMC article.
-
Tolerability of Current Treatments for Dry Eye Disease: A Review of Approved and Investigational Therapies.Clin Ophthalmol. 2024 Aug 16;18:2283-2302. doi: 10.2147/OPTH.S465143. eCollection 2024. Clin Ophthalmol. 2024. PMID: 39165367 Free PMC article. Review.
-
Melanocortin 3,5 receptors immunohistochemical expression in colonic mucosa of inflammatory bowel disease patients: A matter of disease activity?World J Gastroenterol. 2024 Mar 7;30(9):1132-1142. doi: 10.3748/wjg.v30.i9.1132. World J Gastroenterol. 2024. PMID: 38577176 Free PMC article. Clinical Trial.
References
-
- Santiago AR, Pereira TS, Garrido MJ, et al High glucose and diabetes increase the release of [3H]‐D‐aspartate in retinal cell cultures and in rat retinas. Neurochem Int. 2006; 48: 453–8. - PubMed
-
- Behl T, Kaur I, Kotwani A. Implication of oxidative stress in progression of diabetic retinopathy. Surv Ophthalmol. 2016; 61: 187–96. - PubMed
-
- Muriach M, Bosch‐Morell F, Alexander G, et al Lutein effect on retina and hippocampus of diabetic mice. Free Radic Biol Med. 2006; 41: 979–84. - PubMed
-
- Johnsen‐Soriano S, Garcia‐Pous M, Arnal E, et al Early lipoic acid intake protects retina of diabetic mice. Free Radic Res. 2008; 42: 613–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

