Randomized Phase II Trial of Seribantumab in Combination With Paclitaxel in Patients With Advanced Platinum-Resistant or -Refractory Ovarian Cancer
- PMID: 27998236
- PMCID: PMC5562430
- DOI: 10.1200/JCO.2016.67.1891
Randomized Phase II Trial of Seribantumab in Combination With Paclitaxel in Patients With Advanced Platinum-Resistant or -Refractory Ovarian Cancer
Abstract
Purpose Seribantumab is a fully human immunoglobulin G2 monoclonal antibody that binds to human epidermal growth factor receptor (HER) 3 (ErbB3), blocking heregulin (HRG) -mediated ErbB3 signaling and inducing ErbB3 receptor downregulation. This open-label randomized phase II study evaluated progression-free survival (PFS) with seribantumab in combination with once-per-week paclitaxel compared with paclitaxel alone in patients with platinum-resistant or -refractory ovarian cancer. A key secondary objective was to determine if any of five prespecified biomarkers predicted benefit from seribantumab. Patients and Methods Patients with platinum-resistant or -refractory epithelial ovarian, fallopian tube, or primary peritoneal cancer were randomly assigned at a ratio of two to one to receive seribantumab plus paclitaxel or paclitaxel alone. Patients underwent pretreatment core needle biopsy; archival tumor samples were also obtained to support biomarker analyses. Results A total of 223 patients were randomly assigned (seribantumab plus paclitaxel, n = 140; paclitaxel alone, n = 83). Median PFS in the unselected intent-to-treat population was 3.75 months with seribantumab plus paclitaxel compared with 3.68 months with paclitaxel alone (hazard ratio [HR], 1.027; 95% CI, 0.741 to 1.425; P = .864). Among patients whose tumors had detectable HRG mRNA and low HER2 (n = 57 [38%] of 151 with available biomarker data), increased treatment benefit was observed in those receiving seribantumab plus paclitaxel compared with paclitaxel alone (PFS HR, 0.37; 95% CI, 0.18 to 0.76; P = .007). The HR in patients not meeting these criteria was 1.80 (95% CI, 1.08 to 2.98; P = .023). Conclusion The addition of seribantumab to paclitaxel did not result in improved PFS in unselected patients. Exploratory analyses suggest that detectable HRG and low HER2, biomarkers that link directly to the mechanism of action of seribantumab, identified patients who might benefit from this combination. Future clinical trials are needed to validate this finding and should preselect for HRG expression and focus on cancers with low HER2 levels.
Trial registration: ClinicalTrials.gov NCT01447706.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at
Figures
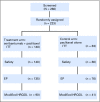



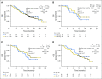

Comment in
-
Picking the Right Patient for Human Epidermal Growth Factor Receptor 3-Targeted Therapy in Platinum-Resistant Ovarian Cancer.J Clin Oncol. 2016 Dec 20;34(36):4312-4314. doi: 10.1200/JCO.2016.69.7169. Epub 2016 Oct 23. J Clin Oncol. 2016. PMID: 27998226 Free PMC article. No abstract available.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. - PubMed
-
- Naumann RW, Coleman RL. Management strategies for recurrent platinum-resistant ovarian cancer. Drugs. 2011;71:1397–1412. - PubMed
-
- Lortholary A, Largillier R, Weber B, et al. Weekly paclitaxel as a single agent or in combination with carboplatin or weekly topotecan in patients with resistant ovarian cancer: The CARTAXHY randomized phase II trial from Groupe d’Investigateurs Nationaux pour l’Etude des Cancers Ovariens (GINECO) Ann Oncol. 2012;23:346–352. - PubMed
-
- Poveda AM, Selle F, Hilpert F, et al. Bevacizumab combined with weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan in platinum-resistant recurrent ovarian cancer: Analysis by chemotherapy cohort of the randomized phase III AURELIA trial. J Clin Oncol. 2015;33:3836–3838. - PubMed
-
- Karlan BY, Oza AM, Richardson GE, et al. Randomized, double-blind, placebo-controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancer. J Clin Oncol. 2012;30:362–371. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

