Directed differentiation of human iPSC into insulin producing cells is improved by induced expression of PDX1 and NKX6.1 factors in IPC progenitors
- PMID: 27998294
- PMCID: PMC5168869
- DOI: 10.1186/s12967-016-1097-0
Directed differentiation of human iPSC into insulin producing cells is improved by induced expression of PDX1 and NKX6.1 factors in IPC progenitors
Abstract
Background: Induced pluripotent stem cells (iPSC) possess an enormous potential as both, scientific and therapeutic tools. Their application in the regenerative medicine provides new treatment opportunities for numerous diseases, including type 1 diabetes. In this work we aimed to derive insulin producing cells (IPC) from iPS cells established in defined conditions.
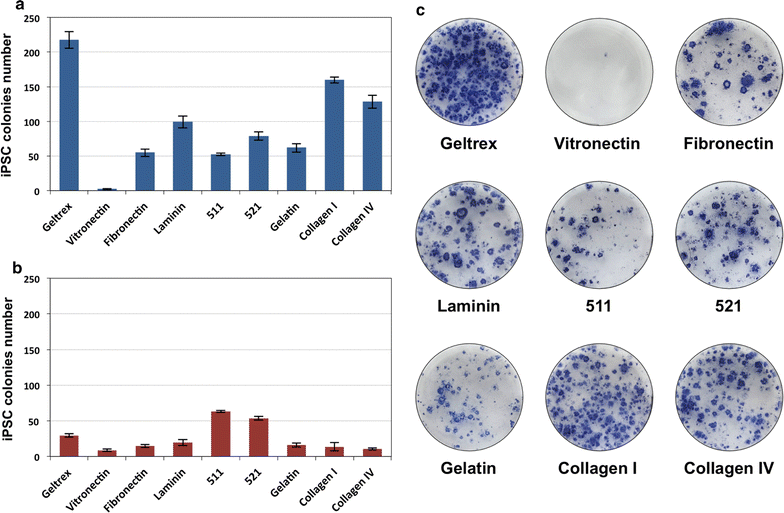
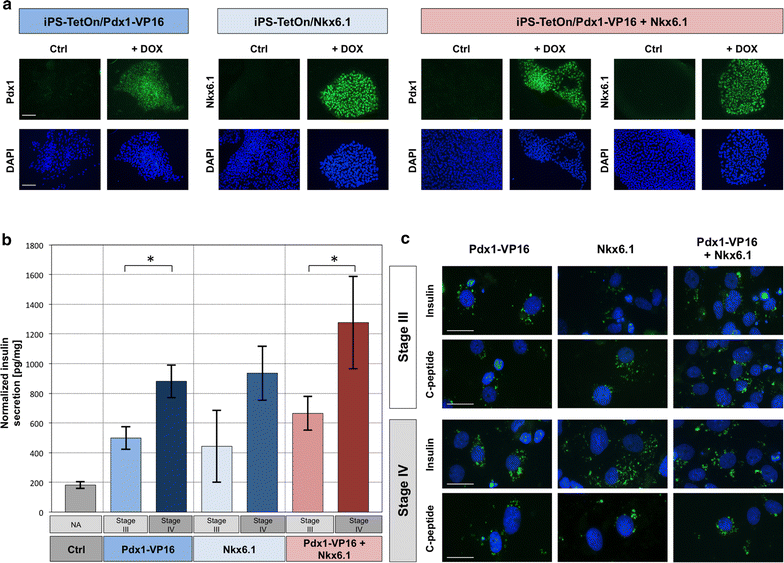
Methods: We optimized iPSC generation protocol and created pluripotent cell lines with stably integrated PDX1 and NKX6.1 transgenes under the transcriptional control of doxycycline-inducible promoter. These cells were differentiated using small chemical molecules and recombinant Activin A in the sequential process through the definitive endoderm, pancreatic progenitor cells and insulin producing cells. Efficiency of the procedure was assessed by quantitative gene expression measurements, immunocytochemical stainings and functional assays for insulin secretion.
Results: Generated cells displayed molecular markers characteristic for respective steps of the differentiation. The obtained IPC secreted insulin and produced C-peptide with significantly higher hormone release level in case of the combined expression of PDX1 and NKX6.1 induced at the last stage of the differentiation.
Conclusions: Efficiency of differentiation of iPSC to IPC can be increased by concurrent expression of PDX1 and NKX6.1 during progenitor cells maturation. Protocols established in our study allow for iPSC generation and derivation of IPC in chemically defined conditions free from animal-derived components, which is of the utmost importance in the light of their prospective applications in the field of regenerative medicine.
Keywords: Defined culture conditions; Diabetes; Differentiation; Induced pluripotent stem cells; Insulin producing cells; NKX6.1; PDX1; Reprogramming.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

