Mechanisms of modulation of brain microvascular endothelial cells function by thrombin
- PMID: 27998795
- PMCID: PMC5350626
- DOI: 10.1016/j.brainres.2016.12.011
Mechanisms of modulation of brain microvascular endothelial cells function by thrombin
Abstract
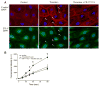
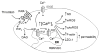
Brain microvascular endothelial cells are a critical component of the blood-brain barrier. They form a tight monolayer which is essential for maintaining the brain homeostasis. Blood-derived proteases such as thrombin may enter the brain during pathological conditions like trauma, stroke, and inflammation and further disrupts the permeability of the blood-brain barrier, via incompletely characterized mechanisms. We examined the underlying mechanisms evoked by thrombin in rat brain microvascular endothelial cells (RBMVEC). Our results indicate that thrombin, acting on protease-activated receptor 1 (PAR1) increases cytosolic Ca2+ concentration in RBMVEC via Ca2+ release from endoplasmic reticulum through inositol 1,4,5-trisphosphate receptors and Ca2+ influx from extracellular space. Thrombin increases nitric oxide production; the effect is abolished by inhibition of the nitric oxide synthase or by antagonism of PAR1 receptors. In addition, thrombin increases mitochondrial and cytosolic reactive oxygen species production via PAR1-dependent mechanisms. Immunocytochemistry studies indicate that thrombin increases F-actin stress fibers, and disrupts the tight junctions. Thrombin increased the RBMVEC permeability assessed by a fluorescent flux assay. Taken together, our results indicate multiple mechanisms by which thrombin modulates the function of RBMVEC and may contribute to the blood-brain barrier dysfunction.
Keywords: Blood-brain barrier; Calcium signaling; Cerebral microvasculature; Protease-activated receptor 1.
Copyright © 2016 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures



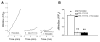
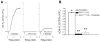
References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
-
- Bartha K, Domotor E, Lanza F, Adam-Vizi V, Machovich R. Identification of thrombin receptors in rat brain capillary endothelial cells. J Cereb Blood Flow Metab. 2000;20:175–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

