Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: a systematic review and meta-analysis of randomized controlled trials
- PMID: 27998966
- PMCID: PMC5834146
- DOI: 10.1093/annonc/mdw673
Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: a systematic review and meta-analysis of randomized controlled trials
Abstract
Background: Aromatase inhibitors (AIs) have been associated with cardiovascular disease in adjuvant randomized controlled trials (RCTs) comparing these drugs to tamoxifen. However, it is unclear whether this risk is real or due to cardioprotective effects of tamoxifen. To address this question, we conducted a systematic review and meta-analysis of all RCTs of AIs and tamoxifen in adjuvant and extended adjuvant setting.
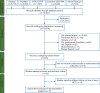
Patients and methods: We searched PubMed, Embase (OVID), Cochrane CENTRAL, WHO International Clinical Trials Registry Platform, and ClinicalTrials.gov from inception to June 2016 for all RCTs comparing cardiovascular and cerebrovascular safety of AIs to tamoxifen, AIs to placebo or no-treatment, or tamoxifen to placebo or no-treatment in the adjuvant or extended adjuvant setting. Relative risks (RRs) were pooled using DerSimonian and Laird random-effects models with analyses stratified by RCT design.
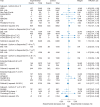
Results: A total of 19 RCTs were included in the meta-analysis (n = 62 345). In the adjuvant setting, AIs were associated with a 19% (RR: 1.19, 95% confidence interval [CI]: 1.07-1.34) increased risk of cardiovascular events compared with tamoxifen. AIs were not associated with an increased risk compared with placebo in the extended-adjuvant setting (RR: 1.01, 95% CI: 0.85-1.20). In the adjuvant setting, tamoxifen was associated with a 33% (RR: 0.67, 95% CI: 0.45-0.98) decreased risk compared with placebo or no-treatment. The results from extended adjuvant RCTs comparing tamoxifen to placebo were inconclusive but suggestive of a small protective effect (RR: 0.91, 95% CI: 0.77-1.07).
Conclusions: The increased risk of cardiovascular events with AIs relative to tamoxifen is likely the result of cardioprotective effects of the latter. This new evidence should be considered when assessing the benefits and risks of AIs in the treatment of breast cancer.
Keywords: anastrozole; aromatase inhibitors; breast cancer; exemestane; letrozole; tamoxifen.
© The Author 2016. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.Health Technol Assess. 2007 Jul;11(26):iii-iv, ix-xi, 1-134. doi: 10.3310/hta11260. Health Technol Assess. 2007. PMID: 17610808
-
Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003370. doi: 10.1002/14651858.CD003370.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2009 Oct 07;(4):CD003370. doi: 10.1002/14651858.CD003370.pub3. PMID: 17253488 Updated.
-
Systematic review of aromatase inhibitors in the first-line treatment for hormone sensitive advanced or metastatic breast cancer.Breast Cancer Res Treat. 2010 Aug;123(1):9-24. doi: 10.1007/s10549-010-0974-0. Epub 2010 Jun 10. Breast Cancer Res Treat. 2010. PMID: 20535542
-
Systemic therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer.Cochrane Database Syst Rev. 2022 Jan 10;1(1):CD013167. doi: 10.1002/14651858.CD013167.pub2. Cochrane Database Syst Rev. 2022. PMID: 35005781 Free PMC article.
-
Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women.Cochrane Database Syst Rev. 2009 Oct 7;2009(4):CD003370. doi: 10.1002/14651858.CD003370.pub3. Cochrane Database Syst Rev. 2009. PMID: 19821307 Free PMC article.
Cited by
-
Gender Differences in Diagnosis, Prevention, and Treatment of Cardiotoxicity in Cardio-Oncology.J Clin Med. 2022 Sep 1;11(17):5167. doi: 10.3390/jcm11175167. J Clin Med. 2022. PMID: 36079097 Free PMC article. Review.
-
Breast cancer and incident cardiovascular events: A systematic analysis at the nationwide level.Eur J Clin Invest. 2022 Jun;52(6):e13754. doi: 10.1111/eci.13754. Epub 2022 Feb 10. Eur J Clin Invest. 2022. PMID: 35113450 Free PMC article.
-
The Role of Cardioprotection in Cancer Therapy Cardiotoxicity: JACC: CardioOncology State-of-the-Art Review.JACC CardioOncol. 2022 Mar 15;4(1):19-37. doi: 10.1016/j.jaccao.2022.01.101. eCollection 2022 Mar. JACC CardioOncol. 2022. PMID: 35492815 Free PMC article. Review.
-
Key metabolic parameters change significantly in early breast cancer survivors: an explorative PILOT study.J Transl Med. 2019 Apr 1;17(1):105. doi: 10.1186/s12967-019-1850-2. J Transl Med. 2019. PMID: 30935397 Free PMC article.
-
Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association.Circulation. 2018 Feb 20;137(8):e30-e66. doi: 10.1161/CIR.0000000000000556. Epub 2018 Feb 1. Circulation. 2018. PMID: 29437116 Free PMC article.
References
-
- Kelly E, Lu CY, Albertini S, Vitry A.. Longitudinal trends in utilization of endocrine therapies for breast cancer: an international comparison. J Clin Pharm Ther 2015; 40: 76–82. - PubMed
-
- Early Breast Cancer Trialists' Collaborative G, Dowsett M, Forbes JF. et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015; 386: 1341–1352. - PubMed
-
- Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol 2006; 7: 633–643. - PubMed
-
- Bliss JM, Kilburn LS, Coleman RE. et al. Disease-related outcomes with long-term follow-up: an updated analysis of the intergroup exemestane study. J Clin Oncol 2012; 30: 709–717. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

