Leptin-Notch signaling axis is involved in pancreatic cancer progression
- PMID: 27999190
- PMCID: PMC5352357
- DOI: 10.18632/oncotarget.13946
Leptin-Notch signaling axis is involved in pancreatic cancer progression
Abstract
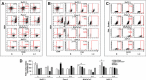
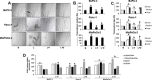
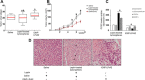
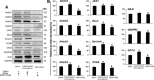
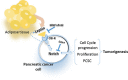
Pancreatic cancer (PC) shows a high death rate. PC incidence and prognosis are affected by obesity, a pandemic characterized by high levels of leptin. Notch is upregulated by leptin in breast cancer. Thus, leptin and Notch crosstalk could influence PC progression. Here we investigated in PC cell lines (BxPC-3, MiaPaCa-2, Panc-1, AsPC-1), derived tumorspheres and xenografts whether a functional leptin-Notch axis affects PC progression and expansion of pancreatic cancer stem cells (PCSC). PC cells and tumorspheres were treated with leptin and inhibitors of Notch (gamma-secretase inhibitor, DAPT) and leptin (iron oxide nanoparticle-leptin peptide receptor antagonist 2, IONP-LPrA2). Leptin treatment increased cell cycle progression and proliferation, and the expression of Notch receptors, ligands and targeted molecules (Notch1-4, DLL4, JAG1, Survivin and Hey2), PCSC markers (CD24/CD44/ESA, ALDH, CD133, Oct-4), ABCB1 protein, as well as tumorsphere formation. Leptin-induced effects on PC and tumorspheres were decreased by IONP-LPrA2 and DAPT. PC cells secreted leptin and expressed the leptin receptor, OB-R, which indicates a leptin autocrine/paracrine signaling loop could also affect tumor progression. IONP-LPrA2 treatment delayed the onset of MiaPaCa-2 xenografts, and decreased tumor growth and the expression of proliferation and PCSC markers. Present data suggest that leptin-Notch axis is involved in PC. PC has no targeted therapy and is mainly treated with chemotherapy, whose efficiency could be decreased by leptin and Notch activities. Thus, the leptin-Notch axis could be a novel therapeutic target, particularly for obese PC patients.
Keywords: Notch; leptin; leptin peptide receptor antagonist LPrA2; pancreatic cancer; pancreatic cancer stem cells.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures


References
-
- American Cancer Society. Cancer facts & Figures. 2016. http://www.cancer.org/acs/groups/content/@research/documents/document/ac...
-
- Amundadottir L, Kaft P, Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA, H Bas Bueno-de-Mesquita, Gross M, Helzlsouer K, Jacobs EJ, LaCroix A, Zheng W, Albanes D, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. 2009;41:986–90. - PMC - PubMed
-
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

