Simultaneous Determination of Multiple Components in Guanjiekang in Rat Plasma via the UPLC-MS/MS Method and Its Application in Pharmacokinetic Study
- PMID: 27999285
- PMCID: PMC6272869
- DOI: 10.3390/molecules21121732
Simultaneous Determination of Multiple Components in Guanjiekang in Rat Plasma via the UPLC-MS/MS Method and Its Application in Pharmacokinetic Study
Abstract
Guanjiekang (GJK) that is formed by five medicinal herbs including Astragali Radix, Aconiti Lateralis Radix Praeparaia, Glycyrrhizae Radix et Rhizoma, Corydalis Rhizoma and Paeoniae Radix Alba was used for the treatment of rheumatoid arthritis (RA). However, the pharmacokinetic (PK) profile of active components in GJK remains unclear. This study aims to evaluate the pharmacokinetic behavior of seven representative active constituents in GJK (i.e., benzoylhypaconine, benzoylmesaconine, paeoniflorin, tetrahydropalmatine, calycosin-7-glucoside, formononetin and isoliquiritigenin) after oral administration of GJK in rats. A rapid, sensitive and reliable ultra-performance liquid chromatography-tandem mass spectrometer (UPLC-MS/MS) method has been successfully developed for the simultaneous determination of these seven constituents in rat plasma. Chromatographic separation was achieved on a C18 column with a gradient elution program that consists of acetonitrile and water (containing 0.1% formic acid) at a flow rate of 0.35 mL/min. Detection was performed under the multiple reaction monitoring (MRM) in the positive electrospray ionization (ESI) mode. The calibration curves exhibited good linearity (R² > 0.99) over a wide concentration range for all constituents. The accuracies ranged from 92.9% to 107.8%, and the intra-day and inter-day precisions at three different levels were below 15%. Our PK results showed that these seven compounds were quickly absorbed after the administration of the GJK product, and Tmax ranged from 30 min to 189 min. The in vivo concentrations of paeoniflorin and isoliquiritigenin were significantly higher than the reported in vitro effective doses, indicating that they could partly contribute to the therapeutic effect of GJK. Therefore, we conclude that pharmacokinetic studies of representative bioactive chemicals after administration of complex herbal products are not only necessary but also feasible. Moreover, these seven compounds that were absorbed in vivo can be used as indicator standards for quality control and for determining pharmacokinetic behavior of herbal medicines in clinical studies.
Keywords: Guanjiekang (GJK); UPLC–MS/MS method; pharmacokinetic study; simultaneous determination of multi-components.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
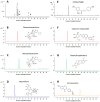
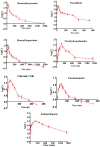
References
-
- Buriani A., Garcia-Bermejo M.L., Bosisio E., Xu Q., Li H., Dong X., Simmonds M.S., Carrara M., Tejedor N., Lucio-Cazana J., et al. Omic techniques in systems biology approaches to traditional chinese medicine research: Present and future. J. Ethnopharmacol. 2012;140:535–544. doi: 10.1016/j.jep.2012.01.055. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

