Opportunities and Challenges for Drug Development: Public-Private Partnerships, Adaptive Designs and Big Data
- PMID: 27999543
- PMCID: PMC5138214
- DOI: 10.3389/fphar.2016.00461
Opportunities and Challenges for Drug Development: Public-Private Partnerships, Adaptive Designs and Big Data
Abstract
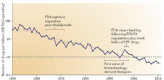
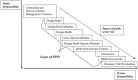
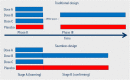
Drug development faces the double challenge of increasing costs and increasing pressure on pricing. To avoid that lack of perceived commercial perspective will leave existing medical needs unmet, pharmaceutical companies and many other stakeholders are discussing ways to improve the efficiency of drug Research and Development. Based on an international symposium organized by the Medical School of the University of Duisburg-Essen (Germany) and held in January 2016, we discuss the opportunities and challenges of three specific areas, i.e., public-private partnerships, adaptive designs and big data. Public-private partnerships come in many different forms with regard to scope, duration and type and number of participants. They range from project-specific collaborations to strategic alliances to large multi-party consortia. Each of them offers specific opportunities and faces distinct challenges. Among types of collaboration, investigator-initiated studies are becoming increasingly popular but have legal, ethical, and financial implications. Adaptive trial designs are also increasingly discussed. However, adaptive should not be used as euphemism for the repurposing of a failed trial; rather it requires carefully planning and specification before a trial starts. Adaptive licensing can be a counter-part of adaptive trial design. The use of Big Data is another opportunity to leverage existing information into knowledge useable for drug discovery and development. Respecting limitations of informed consent and privacy is a key challenge in the use of Big Data. Speakers and participants at the symposium were convinced that appropriate use of the above new options may indeed help to increase the efficiency of future drug development.
Keywords: adaptive trial design; big data; drug development; informed consent; investigator-initiated studies; privacy; public–private partnership.
Figures
References
-
- Anonymous (2015). 2015 Editor of the CRO Market Size Projections: 2012-2019. Available at: http://www.isrreports.com
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

