IL-1 Inhibition in Systemic Juvenile Idiopathic Arthritis
- PMID: 27999545
- PMCID: PMC5138234
- DOI: 10.3389/fphar.2016.00467
IL-1 Inhibition in Systemic Juvenile Idiopathic Arthritis
Abstract
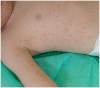
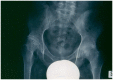
Systemic juvenile idiopathic arthritis (sJIA) is the form of childhood arthritis whose treatment is most challenging. The demonstration of the prominent involvement of interleukin (IL)-1 in disease pathogenesis has provided the rationale for the treatment with biologic medications that antagonize this cytokine. The three IL-1 blockers that have been tested so far (anakinra, canakinumab, and rilonacept) have all been proven effective and safe, although only canakinumab is currently approved for use in sJIA. The studies on IL-1 inhibition in sJIA published in the past few years suggest that children with fewer affected joints, higher neutrophil count, younger age at disease onset, shorter disease duration, or, possibly, higher ferritin level may respond better to anti-IL-1 treatment. In addition, it has been postulated that use of IL-1 blockade as first-line therapy may take advantage of a "window of opportunity," in which disease pathophysiology can be altered to prevent the occurrence of chronic arthritis. In this review, we analyze the published literature on IL-1 inhibitors in sJIA and discuss the rationale underlying the use of these medications, the results of therapeutic studies, and the controversial issues.
Keywords: IL1-inhibitors; anakinra; canakinumab; rilonacept; systemic juvenile idiopathic arthritis.
Figures

References
-
- Behrens E. M., Beukelman T., Gallo L., Spangler J., Rosenkranz M., Arkachaisri T., et al. . (2008). Evaluation of the presentation of systemic onset juvenile rheumatoid arthritis: data from the Pennsylvania Systemic Onset Juvenile Arthritis Registry (PASOJAR). J. Rheumatol. 35, 343–348. - PubMed
-
- Behrens E. M., Beukelman T., Paessler M., Cron R. Q. (2007). Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J. Rheumatol. 34, 1133–1138. - PubMed
-
- Bleesing J., Prada A., Siegel D. M., Villanueva J., Olson J., Ilowite N. T., et al. . (2007). The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor alpha-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 56, 965–971. 10.1002/art.22416 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

