Immune response and long-term clinical outcome in advanced melanoma patients vaccinated with tumor-mRNA-transfected dendritic cells
- PMID: 27999747
- PMCID: PMC5139630
- DOI: 10.1080/2162402X.2016.1232237
Immune response and long-term clinical outcome in advanced melanoma patients vaccinated with tumor-mRNA-transfected dendritic cells
Abstract
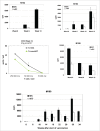
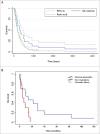
The most effective anticancer immune responses are probably directed against patient-specific neoantigens. We have developed a melanoma vaccine targeting this individual mutanome based on dendritic cells (DCs) loaded with autologous tumor-mRNA. Here, we report a phase I/II trial evaluating toxicity, immune response and clinical outcome in 31 metastatic melanoma patients. The first cohort (n = 22) received the vaccine without any adjuvant; the next cohort (n = 9) received adjuvant IL2. Each subject received four weekly intranodal or intradermal injections, followed by optional monthly vaccines. Immune response was evaluated by delayed-type hypersensitivity (DTH), T cell proliferation and cytokine assays. Data were collected for 10 y after inclusion of the last patient. No serious adverse events were detected. In the intention-to-treat-cohort, we demonstrated significantly superior survival compared to matched controls from a benchmark meta-analysis (1 y survival 43% vs. 24%, 2 y 23% vs. 6.6%). A tumor-specific immune response was demonstrated in 16/31 patients. The response rate was higher after intradermal than intranodal vaccination (80% vs. 38%). Immune responders had improved survival compared to non-responders (median 14 mo vs. 6 mo; p = 0.030), and all eight patients surviving >20 mo were immune responders. In addition to the tumor-specific response, most patients developed a response against autologous DC antigens. The cytokine profile was polyfunctional and did not follow a Th1/Th2 dichotomy. We conclude that the favorable safety profile and evidence of a possible survival benefit warrant further studies of the RNA/DC vaccine. The vaccine appears insufficient as monotherapy, but there is a strong rationale for combination with checkpoint modulators.
Keywords: Cancer vaccine; T cell; clinical outcome; dendritic cell; mRNA; melanoma; survival.
Figures



Similar articles
-
Phase I/II trial of melanoma therapy with dendritic cells transfected with autologous tumor-mRNA.Cancer Gene Ther. 2006 Oct;13(10):905-18. doi: 10.1038/sj.cgt.7700961. Epub 2006 May 5. Cancer Gene Ther. 2006. PMID: 16710345 Clinical Trial.
-
Randomized phase II trial of autologous dendritic cell vaccines versus autologous tumor cell vaccines in metastatic melanoma: 5-year follow up and additional analyses.J Immunother Cancer. 2018 Mar 6;6(1):19. doi: 10.1186/s40425-018-0330-1. J Immunother Cancer. 2018. PMID: 29510745 Free PMC article. Clinical Trial.
-
Route of administration modulates the induction of dendritic cell vaccine-induced antigen-specific T cells in advanced melanoma patients.Clin Cancer Res. 2011 Sep 1;17(17):5725-35. doi: 10.1158/1078-0432.CCR-11-1261. Epub 2011 Jul 19. Clin Cancer Res. 2011. PMID: 21771874
-
Dendritic cell gene therapy.Surg Oncol Clin N Am. 2002 Jul;11(3):645-60. doi: 10.1016/s1055-3207(02)00027-3. Surg Oncol Clin N Am. 2002. PMID: 12487060 Review.
-
Immune modulations during chemoimmunotherapy & novel vaccine strategies--in metastatic melanoma and non small-cell lung cancer.Dan Med J. 2013 Dec;60(12):B4774. Dan Med J. 2013. PMID: 24355457 Review.
Cited by
-
NPM-ALK-reactive T-cell responses in children and adolescents with NPM-ALK positive anaplastic large cell lymphoma.Oncoimmunology. 2019 Jun 26;8(9):e1625688. doi: 10.1080/2162402X.2019.1625688. eCollection 2019. Oncoimmunology. 2019. PMID: 31428523 Free PMC article.
-
Ribonucleic Acid Engineering of Dendritic Cells for Therapeutic Vaccination: Ready 'N Able to Improve Clinical Outcome?Cancers (Basel). 2020 Jan 27;12(2):299. doi: 10.3390/cancers12020299. Cancers (Basel). 2020. PMID: 32012714 Free PMC article. Review.
-
Dendritic Cell Vaccines: A Shift from Conventional Approach to New Generations.Cells. 2023 Aug 25;12(17):2147. doi: 10.3390/cells12172147. Cells. 2023. PMID: 37681880 Free PMC article. Review.
-
Individualized Neoantigen-Directed Melanoma Therapy.Am J Clin Dermatol. 2025 Mar;26(2):225-235. doi: 10.1007/s40257-025-00920-4. Epub 2025 Jan 29. Am J Clin Dermatol. 2025. PMID: 39875711 Review.
-
Advancements in Melanoma Therapies: From Surgery to Immunotherapy.Curr Treat Options Oncol. 2024 Aug;25(8):1073-1088. doi: 10.1007/s11864-024-01239-8. Epub 2024 Jul 27. Curr Treat Options Oncol. 2024. PMID: 39066854 Review.
References
-
- Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, Moon J, Sondak VK, Atkins MB, Eisenhauer EA et al.. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 2008; 26(4):527-34; PMID:18235113; http://dx.doi.org/10.1200/JCO.2007.12.7837 - DOI - PubMed
-
- Eigentler TK, Caroli UM, Radny P, Garbe C. Palliative therapy of disseminated malignant melanoma: a systematic review of 41 randomised clinical trials. Lancet Oncol 2003; 4(12):748-59; PMID:14662431; http://dx.doi.org/10.1016/S1470-2045(03)01280-4 - DOI - PubMed
-
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363(8):711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466 - DOI - PMC - PubMed
-
- Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC et al.. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014; 384(9948):1109-17; PMID:25034862; http://dx.doi.org/10.1016/S0140-6736(14)60958-2 - DOI - PubMed
-
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P et al.. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373(1):23-34; PMID:26027431; http://dx.doi.org/10.1056/NEJMoa1504030 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
