Functional impairment of infiltrating T cells in human colorectal cancer
- PMID: 27999752
- PMCID: PMC5139627
- DOI: 10.1080/2162402X.2016.1234573
Functional impairment of infiltrating T cells in human colorectal cancer
Abstract
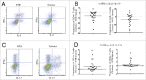
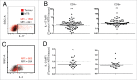
T cells play a crucial role in preventing the growth and spread of colorectal cancer (CRC). However, immunotherapies against CRC have only shown limited success, which may be due to lack of understanding about the effect of the local tumor microenvironment (TME) on T cell function. The goal of this study was to determine whether T cells in tumor tissue were functionally impaired compared to T cells in non-tumor bowel (NTB) tissue from the same patients. We showed that T cell populations are affected differently by the TME. In the tumor, T cells produced more IL-17 and less IL-2 per cell than their counterparts from NTB tissue. T cells from tumor tissue also had impaired proliferative ability compared to T cells in NTB tissue. This impairment was not related to the frequency of IL-2 producing T cells or regulatory T cells, but T cells from the TME had a higher co-expression of inhibitory receptors than T cells from NTB. Overall, our data indicate that T cells in tumor tissue are functionally altered by the CRC TME, which is likely due to cell intrinsic factors. The TME is therefore an important consideration in predicting the effect of immune modulatory therapies.
Keywords: Colorectal cancer; IL-2; T cells; exhaustion; proliferation.
Figures



References
-
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2016:1-9; PMID:26187505; http://dx.doi.org/10.1136/gutjnl-2015-310912 - DOI - PubMed
-
- Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A et al.. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013; 39:782-95; PMID:24138885; http://dx.doi.org/10.1016/j.immuni.2013.10.003 - DOI - PubMed
-
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D et al.. Effector memory T-cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353:2654-66; PMID:16371631; http://dx.doi.org/10.1056/NEJMoa051424 - DOI - PubMed
-
- Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C et al.. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol 2014; 232:199-209; PMID:24122236; http://dx.doi.org/10.1002/path.4287 - DOI - PMC - PubMed
-
- Amin M, Lockhart AC. The potential role of immunotherapy to treat colorectal cancer. Expert Opin Inv Drugs 2015; 24:329-44; http://dx.doi.org/10.1517/13543784.2015.985376 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
