ExsE Is a Negative Regulator for T3SS Gene Expression in Vibrio alginolyticus
- PMID: 27999769
- PMCID: PMC5138213
- DOI: 10.3389/fcimb.2016.00177
ExsE Is a Negative Regulator for T3SS Gene Expression in Vibrio alginolyticus
Abstract
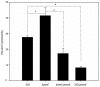
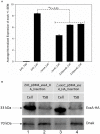
Type III secretion systems (T3SSs) contribute to microbial pathogenesis of Vibrio species, but the regulatory mechanisms are complex. We determined if the classic ExsACDE protein-protein regulatory model from Pseudomonas aeruginosa applies to Vibrio alginolyticus. Deletion mutants in V. alginolyticus demonstrated that, as expected, the T3SS is positively regulated by ExsA and ExsC and negatively regulated by ExsD and ExsE. Interestingly, deletion of exsE enhanced the ability of V. alginolyticus to induce host-cell death while cytotoxicity was inhibited by in trans complementation of this gene in a wild-type strain, a result that differs from a similar experiment with Vibrio parahaemolyticus ExsE. We further showed that ExsE is a secreted protein that does not contribute to adhesion to Fathead minnow epithelial cells. An in vitro co-immunoprecipitation assay confirmed that ExsE binds to ExsC to exert negative regulatory effect on T3SS genes. T3SS in V. alginolyticus can be activated in the absence of physical contact with host cells and a separate regulatory pathway appears to contribute to the regulation of ExsA. Consequently, like ExsE from P. aeruginosa, ExsE is a negative regulator for T3SS gene expression in V. alginolyticus. Unlike the V. parahaemolyticus orthologue, however, deletion of exsE from V. alginolyticus enhanced in vitro cytotoxicity.
Keywords: ExsACDE; ExsE; T3SS; Vibrio alginolyticus; gene expression; negative regulator.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

