Flash Glucose-Sensing Technology as a Replacement for Blood Glucose Monitoring for the Management of Insulin-Treated Type 2 Diabetes: a Multicenter, Open-Label Randomized Controlled Trial
- PMID: 28000140
- PMCID: PMC5306122
- DOI: 10.1007/s13300-016-0223-6
Flash Glucose-Sensing Technology as a Replacement for Blood Glucose Monitoring for the Management of Insulin-Treated Type 2 Diabetes: a Multicenter, Open-Label Randomized Controlled Trial
Abstract
Introduction: Glycemic control in participants with insulin-treated diabetes remains challenging. We assessed safety and efficacy of new flash glucose-sensing technology to replace self-monitoring of blood glucose (SMBG).
Methods: This open-label randomized controlled study (ClinicalTrials.gov, NCT02082184) enrolled adults with type 2 diabetes on intensive insulin therapy from 26 European diabetes centers. Following 2 weeks of blinded sensor wear, 2:1 (intervention/control) randomization (centrally, using biased-coin minimization dependant on study center and insulin administration) was to control (SMBG) or intervention (glucose-sensing technology). Participants and investigators were not masked to group allocation. Primary outcome was difference in HbA1c at 6 months in the full analysis set. Prespecified secondary outcomes included time in hypoglycemia, effect of age, and patient satisfaction.
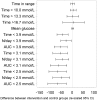
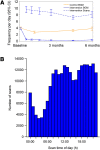
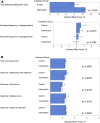
Results: Participants (n = 224) were randomized (149 intervention, 75 controls). At 6 months, there was no difference in the change in HbA1c between intervention and controls: -3.1 ± 0.75 mmol/mol, [-0.29 ± 0.07% (mean ± SE)] and -3.4 ± 1.04 mmol/mol (-0.31 ± 0.09%) respectively; p = 0.8222. A difference was detected in participants aged <65 years [-5.7 ± 0.96 mmol/mol (-0.53 ± 0.09%) and -2.2 ± 1.31 mmol/mol (-0.20 ± 0.12%), respectively; p = 0.0301]. Time in hypoglycemia <3.9 mmol/L (70 mg/dL) reduced by 0.47 ± 0.13 h/day [mean ± SE (p = 0.0006)], and <3.1 mmol/L (55 mg/dL) reduced by 0.22 ± 0.07 h/day (p = 0.0014) for intervention participants compared with controls; reductions of 43% and 53%, respectively. SMBG frequency, similar at baseline, decreased in intervention participants from 3.8 ± 1.4 tests/day (mean ± SD) to 0.3 ± 0.7, remaining unchanged in controls. Treatment satisfaction was higher in intervention compared with controls (DTSQ 13.1 ± 0.50 (mean ± SE) and 9.0 ± 0.72, respectively; p < 0.0001). No serious adverse events or severe hypoglycemic events were reported related to sensor data use. Forty-two serious events [16 (10.7%) intervention participants, 12 (16.0%) controls] were not device-related. Six intervention participants reported nine adverse events for sensor-wear reactions (two severe, six moderate, one mild).
Conclusion: Flash glucose-sensing technology use in type 2 diabetes with intensive insulin therapy results in no difference in HbA1c change and reduced hypoglycemia, thus offering a safe, effective replacement for SMBG.
Trial registration: ClinicalTrials.gov identifier: NCT02082184.
Funding: Abbott Diabetes Care.
Keywords: Flash sensor glucose technology; Glucose monitoring; Insulin; Type 2 diabetes.
Figures
Comment in
-
Blood Glucose Monitoring Data Should Be Reported in Detail When Studies About Efficacy of Continuous Glucose Monitoring Systems Are Published.J Diabetes Sci Technol. 2018 Sep;12(5):1061-1063. doi: 10.1177/1932296817753629. Epub 2018 Jan 11. J Diabetes Sci Technol. 2018. PMID: 29322825 Free PMC article.
References
-
- Holman RR, Paul SK, Bethel MA, et al. 10-year follow up of intensive glucose control in type 2 diabetes. N Eng J Med. 2008;359:577–589. - PubMed
-
- UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. doi: 10.1016/S0140-6736(98)07019-6. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

