C-H and C-N Activation at Redox-Active Pyridine Complexes of Iron
- PMID: 28000416
- PMCID: PMC5266524
- DOI: 10.1002/anie.201610679
C-H and C-N Activation at Redox-Active Pyridine Complexes of Iron
Abstract
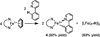
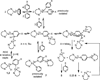
Pyridine activation by inexpensive iron catalysts has great utility, but the steps through which iron species can break the strong (105-111 kcal mol-1 ) C-H bonds of pyridine substrates are unknown. In this work, we report the rapid room-temperature cleavage of C-H bonds in pyridine, 4-tert-butylpyridine, and 2-phenylpyridine by an iron(I) species, to give well-characterized iron(II) products. In addition, 4-dimethylaminopyridine (DMAP) undergoes room-temperature C-N bond cleavage, which forms a dimethylamidoiron(II) complex and a pyridyl-bridged tetrairon(II) square. These facile bond-cleaving reactions are proposed to occur through intermediates having a two-electron reduced pyridine that bridges two iron centers. Thus, the redox non-innocence of the pyridine can play a key role in enabling high regioselectivity for difficult reactions.
Keywords: bond cleavage; iron; pyridine; redox-activity.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




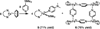
Similar articles
-
Synthesis, electronic structure, and catalytic activity of reduced bis(aldimino)pyridine iron compounds: experimental evidence for ligand participation.Inorg Chem. 2011 Apr 4;50(7):3159-69. doi: 10.1021/ic102186q. Epub 2011 Mar 11. Inorg Chem. 2011. PMID: 21395246
-
Synthesis and ligand non-innocence of thiolate-ligated (N4S) Iron(II) and nickel(II) bis(imino)pyridine complexes.Inorg Chem. 2013 Sep 16;52(18):10467-80. doi: 10.1021/ic4013558. Epub 2013 Aug 30. Inorg Chem. 2013. PMID: 23992096 Free PMC article.
-
Synthesis and electronic structure determination of N-alkyl-substituted bis(imino)pyridine iron imides exhibiting spin crossover behavior.J Am Chem Soc. 2011 Nov 2;133(43):17353-69. doi: 10.1021/ja205736m. Epub 2011 Oct 10. J Am Chem Soc. 2011. PMID: 21985461
-
[CuO](+) and [CuOH](2+) complexes: intermediates in oxidation catalysis?Acc Chem Res. 2015 Jul 21;48(7):2126-31. doi: 10.1021/acs.accounts.5b00169. Epub 2015 Jun 15. Acc Chem Res. 2015. PMID: 26075312 Free PMC article. Review.
-
Dinickel Active Sites Supported by Redox-Active Ligands.Acc Chem Res. 2021 Oct 5;54(19):3710-3719. doi: 10.1021/acs.accounts.1c00424. Epub 2021 Sep 26. Acc Chem Res. 2021. PMID: 34565142 Free PMC article. Review.
Cited by
-
Reversible Ligand-Centered Reduction in Low-Coordinate Iron Formazanate Complexes.Chemistry. 2018 Jul 2;24(37):9417-9425. doi: 10.1002/chem.201801298. Epub 2018 Jun 7. Chemistry. 2018. PMID: 29663542 Free PMC article.
-
Dinitrogen Activation and Functionalization using β-Diketiminate Iron Complexes.Eur J Inorg Chem. 2019 Apr 16;2019(14):1861-1869. doi: 10.1002/ejic.201900133. Epub 2019 Apr 1. Eur J Inorg Chem. 2019. PMID: 31213945 Free PMC article.
-
Synthesis of a Hexameric Magnesium 4-pyridyl Complex with Cyclohexane-like Ring Structure via Reductive C-N Activation.Molecules. 2021 Nov 28;26(23):7214. doi: 10.3390/molecules26237214. Molecules. 2021. PMID: 34885796 Free PMC article.
-
Terminal Hydride Complex of High-Spin Mn.J Am Chem Soc. 2024 Jul 10;146(27):18370-18378. doi: 10.1021/jacs.4c03310. Epub 2024 Jun 28. J Am Chem Soc. 2024. PMID: 38940813 Free PMC article.
-
Alkali Cation Effects on Redox-Active Formazanate Ligands in Iron Chemistry.Inorg Chem. 2018 Aug 20;57(16):9580-9591. doi: 10.1021/acs.inorgchem.8b00226. Epub 2018 Apr 9. Inorg Chem. 2018. PMID: 29629752 Free PMC article.
References
-
- Comins DL, O’Connor S, Al-awar RS. In: Comprehensive Heterocyclic Chemistry III. Taylor AR, Katritzky CA, Ramsden EFV, Scriven RJK, editors. Oxford: Elsevier; 2008. pp. 41–99.
-
- Bull JA, Mousseau JJ, Pelletier G, Charette AB. Chem. Rev. 2012;112:2642–2713. - PubMed
-
- Sadimenko AP. In: Adv. Heterocycl. Chem. Alan RK, editor. Vol. 88. Academic Press; 2005. pp. 111–174.
- Cuesta L, Urriolabeitia EP. Comments Inorg. Chem. 2012;33:55–85.
- Zhu G, Tanski JM, Churchill DG, Janak KE, Parkin G. J. Am. Chem. Soc. 2002;124:13658–13659. - PubMed
- Bradley CA, Lobkovsky E, Chirik PJ. J. Am. Chem. Soc. 2003;125:8110–8111. - PubMed
- Ozerov OV, Pink M, Watson LA, Caulton KG. J. Am. Chem. Soc. 2004;126:2105–2113. - PubMed
- Arndt S, Elvidge BR, Zeimentz PM, Spaniol TP, Okuda J. Organometallics. 2006;25:793–795.
- Lewis JC, Bergman RG, Ellman JA. J. Am. Chem. Soc. 2007;129:5332–5333. - PMC - PubMed
- Esteruelas MA, Forcén E, Oliván M, Oñate E. Organometallics. 2008;27:6188–6192.
- Oishi M, Kato T, Nakagawa M, Suzuki H. Organometallics. 2008;27:6046–6049.
- Berman AM, Lewis JC, Bergman RG, Ellman JA. J. Am. Chem. Soc. 2008;130:14926–14927. - PMC - PubMed
- Carver CT, Diaconescu PL. Journal of Alloys and Compounds. 2009;488:518–523.
- Andino JG, Kilgore UJ, Pink M, Ozarowski A, Krzystek J, Telser J, Baik M-H, Mindiola DJ. Chem. Sci. 2010;1:351–356.
- Tobisu M, Hyodo I, Chatani N. J. Am. Chem. Soc. 2009;131:12070–12071. - PubMed
- Kuppuswamy S, Ghiviriga I, Abboud KA, Veige AS. Organometallics. 2010;29:6711–6722.
- Ye M, Gao G-L, Yu J-Q. J. Am. Chem. Soc. 2011;133:6964–6967. - PubMed
- Nakao Y. Synthesis. 2011;2011:3209–3219.
- Ren S, Xie Z. Organometallics. 2011;30:5953–5959.
- Wicker BF, Fan H, Hickey AK, Crestani MG, Scott J, Pink M, Mindiola DJ. J. Am. Chem. Soc. 2012;134:20081–20096. - PubMed
- Johnson DG, Lynam JM, Mistry NS, Slattery JM, Thatcher RJ, Whitwood AC. J. Am. Chem. Soc. 2013;135:2222–2234. - PubMed
- Han W, Jin F, Zhao Q, Du H, Yao L. Synlett. 2016;27:1854–1859.
-
- Norinder J, Matsumoto A, Yoshikai N, Nakamura E. J. Am. Chem. Soc. 2008;130:5858–5859. - PubMed
- Yoshikai N, Asako S, Yamakawa T, Ilies L, Nakamura E. Chem. Asian J. 2011;6:3059–3065. - PubMed
- Ilies L, Kobayashi M, Matsumoto A, Yoshikai N, Nakamura E. Adv. Synth. Catal. 2012;354:593–596.
- Sun C-L, Li B-J, Shi Z-J. Chem. Rev. 2011;111:1293–1314. - PubMed
- Bauer I, Knölker H-J. Chem. Rev. 2015;115:3170–3387. - PubMed
- Tran LD, Daugulis O. Org. Lett. 2010;12:4277–4279. - PMC - PubMed
- Liu S, Hu X, Li X, Cheng J. Syn. Lett. 2013;24:847–850.
-
- Chisholm MH, Huffman JC, Rothwell IP, Bradley PG, Kress N, Woodruff WH. J. Am. Chem. Soc. 1981;103:4945–4947.
- Fedushkin IL, Petrovskaya TV, Girgsdies F, Kohn RD, Bochkarev MN, Schumann H. Angew. Chem. 1999;111:2407–2409. Angew. Chem., Int. Ed.1999, 38, 2262–2264. - PubMed
- Prabusankar G, Molard Y, Cordier S, Golhen S, Le Gal Y, Perrin C, Ouahab L, Kahlal S, Halet JF. Eur. J. Inorg. Chem. 2009:2153–2161.
- Scarborough CC, Wieghardt K. Inorg. Chem. 2011;50:9773–9793. - PubMed
- Sieh D, Kubiak CP. Chem. Eur. J. 2016;22:10638–10650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

