A crotonyl-CoA reductase-carboxylase independent pathway for assembly of unusual alkylmalonyl-CoA polyketide synthase extender units
- PMID: 28000660
- PMCID: PMC5187497
- DOI: 10.1038/ncomms13609
A crotonyl-CoA reductase-carboxylase independent pathway for assembly of unusual alkylmalonyl-CoA polyketide synthase extender units
Abstract
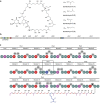
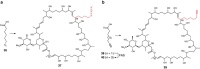
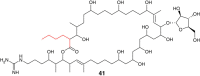
Type I modular polyketide synthases assemble diverse bioactive natural products. Such multienzymes typically use malonyl and methylmalonyl-CoA building blocks for polyketide chain assembly. However, in several cases more exotic alkylmalonyl-CoA extender units are also known to be incorporated. In all examples studied to date, such unusual extender units are biosynthesized via reductive carboxylation of α, β-unsaturated thioesters catalysed by crotonyl-CoA reductase/carboxylase (CCRC) homologues. Here we show using a chemically-synthesized deuterium-labelled mechanistic probe, and heterologous gene expression experiments that the unusual alkylmalonyl-CoA extender units incorporated into the stambomycin family of polyketide antibiotics are assembled by direct carboxylation of medium chain acyl-CoA thioesters. X-ray crystal structures of the unusual β-subunit of the acyl-CoA carboxylase (YCC) responsible for this reaction, alone and in complex with hexanoyl-CoA, reveal the molecular basis for substrate recognition, inspiring the development of methodology for polyketide bio-orthogonal tagging via incorporation of 6-azidohexanoic acid and 8-nonynoic acid into novel stambomycin analogues.
Figures




Similar articles
-
Identification of Middle Chain Fatty Acyl-CoA Ligase Responsible for the Biosynthesis of 2-Alkylmalonyl-CoAs for Polyketide Extender Unit.J Biol Chem. 2015 Nov 6;290(45):26994-27011. doi: 10.1074/jbc.M115.677195. Epub 2015 Sep 16. J Biol Chem. 2015. PMID: 26378232 Free PMC article.
-
Establishing a toolkit for precursor-directed polyketide biosynthesis: exploring substrate promiscuities of acid-CoA ligases.Biochemistry. 2012 Jun 5;51(22):4568-79. doi: 10.1021/bi300425j. Epub 2012 May 22. Biochemistry. 2012. PMID: 22587726
-
Beyond ethylmalonyl-CoA: the functional role of crotonyl-CoA carboxylase/reductase homologs in expanding polyketide diversity.Nat Prod Rep. 2012 Jan;29(1):72-86. doi: 10.1039/c1np00082a. Epub 2011 Nov 29. Nat Prod Rep. 2012. PMID: 22124767 Review.
-
Combining Promiscuous Acyl-CoA Oxidase and Enoyl-CoA Carboxylase/Reductases for Atypical Polyketide Extender Unit Biosynthesis.Cell Chem Biol. 2018 Jul 19;25(7):833-839.e4. doi: 10.1016/j.chembiol.2018.04.009. Epub 2018 May 3. Cell Chem Biol. 2018. PMID: 29731424
-
[Efficient production of polyketide products in Streptomyces hosts - A review].Wei Sheng Wu Xue Bao. 2016 Mar 4;56(3):418-28. Wei Sheng Wu Xue Bao. 2016. PMID: 27382785 Review. Chinese.
Cited by
-
Searching for Glycosylated Natural Products in Actinomycetes and Identification of Novel Macrolactams and Angucyclines.Front Microbiol. 2018 Jan 30;9:39. doi: 10.3389/fmicb.2018.00039. eCollection 2018. Front Microbiol. 2018. PMID: 29441046 Free PMC article.
-
Exploiting the inherent promiscuity of the acyl transferase of the stambomycin polyketide synthase for the mutasynthesis of analogues.Chem Sci. 2025 Jan 22;16(12):5076-5088. doi: 10.1039/d4sc06976e. eCollection 2025 Mar 19. Chem Sci. 2025. PMID: 39886430 Free PMC article.
-
Synthetic Biology in Natural Product Biosynthesis.Chem Rev. 2025 Apr 9;125(7):3814-3931. doi: 10.1021/acs.chemrev.4c00567. Epub 2025 Mar 21. Chem Rev. 2025. PMID: 40116601 Review.
-
Enhanced triacylglycerol metabolism contributes to the efficient biosynthesis of spinosad in Saccharopolyspora spinosa.Synth Syst Biotechnol. 2024 Jun 25;9(4):809-819. doi: 10.1016/j.synbio.2024.06.007. eCollection 2024 Dec. Synth Syst Biotechnol. 2024. PMID: 39072147 Free PMC article.
-
NLRP10 promotes AGEs-induced NLRP1 and NLRP3 inflammasome activation via ROS/MAPK/NF-κB signaling in human periodontal ligament cells.Odontology. 2024 Jan;112(1):100-111. doi: 10.1007/s10266-023-00813-0. Epub 2023 Apr 12. Odontology. 2024. PMID: 37043073
References
-
- Hertweck C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. Engl. 48, 4688–4716 (2009). - PubMed
-
- Song L. et al.. Cytochrome P450-mediated hydroxylation is required for polyketide macrolactonization in stambomycin biosynthesis. J. Antibiot. (Tokyo) 67, 71–76 (2014). - PubMed
-
- Quade N., Huo L., Rachid S., Heinz D. W. & Müller R. Unusual carbon fixation gives rise to diverse polyketide extender units. Nat. Chem. Biol. 8, 117–124 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

