Mycobacterium tuberculosis cell wall released fragments by the action of the human lung mucosa modulate macrophages to control infection in an IL-10-dependent manner
- PMID: 28000679
- PMCID: PMC5479761
- DOI: 10.1038/mi.2016.115
Mycobacterium tuberculosis cell wall released fragments by the action of the human lung mucosa modulate macrophages to control infection in an IL-10-dependent manner
Abstract
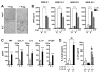
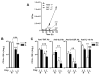
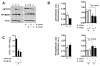
Mycobacterium tuberculosis (M.tb), the causative agent of tuberculosis, is a major public health challenge facing the world. During infection, M.tb is deposited in the lung alveolar space where it comes in contact with the lung mucosa, known as alveolar lining fluid (ALF), an environment that M.tb encounters at different stages of the infection and disease. ALF is abundant in homeostatic and antimicrobial hydrolytic enzymes, also known as hydrolases. Here we demonstrate that ALF hydrolases, at their physiological concentrations and upon contact with M.tb, release M.tb cell envelope fragments into the milieu. These released fragments are bioactive, but non-cytotoxic, regulate the function of macrophages, and thus are capable of modulating the immune response contributing to the control of M.tb infection by human macrophages. Specifically, macrophages exposed to fragments derived from the exposure of M.tb to ALF were able to control the infection primarily by increasing phagosome-lysosome fusion and acidification events. This enhanced control was found to be dependent on fragment-induced interleukin-10 (IL-10) production but also involves the STAT3 signaling pathway in an IL-10-independent manner. Collectively our data indicate that M.tb fragments released upon contact with lung mucosa hydrolases participate in the host immune response to M.tb infection through innate immune modulation.
Conflict of interest statement
The authors declared no conflict of interest.
Figures



References
-
- WHO. WHO Tuberculosis Fact Sheet 2015. World Health Organization; 15 A.D. Mar 20, Available from: http://www.who.int/mediacentre/factsheets/fs104/en/
-
- Torrelles JB. Broadening our view about the role of Mycobacterium tuberculosis cell envelope components during infection: A battle for survival. In: Cardona PJ, editor. Understanding Tuberculosis - Analyzing the Origin of Mycobacterium tuberculosis Pathogenicity. Rijeka, Croatia: Intech; 2012. pp. 77–122.
-
- Schlesinger LS. Phagocytosis and Toll-like Receptors in Tuberculosis. In: Rom WN, Garay SM, editors. Tuberculosis. 2. Lippincott, Williams, & Wilkins; 2004. pp. 203–214.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

