A cell-autonomous tumour suppressor role of RAF1 in hepatocarcinogenesis
- PMID: 28000790
- PMCID: PMC5187498
- DOI: 10.1038/ncomms13781
A cell-autonomous tumour suppressor role of RAF1 in hepatocarcinogenesis
Abstract
Hepatocellular carcinoma (HCC) is a leading cause of cancer deaths, but its molecular heterogeneity hampers the design of targeted therapies. Currently, the only therapeutic option for advanced HCC is Sorafenib, an inhibitor whose targets include RAF. Unexpectedly, RAF1 expression is reduced in human HCC samples. Modelling RAF1 downregulation by RNAi increases the proliferation of human HCC lines in xenografts and in culture; furthermore, RAF1 ablation promotes chemical hepatocarcinogenesis and the proliferation of cultured (pre)malignant mouse hepatocytes. The phenotypes depend on increased YAP1 expression and STAT3 activation, observed in cultured RAF1-deficient cells, in HCC xenografts, and in autochthonous liver tumours. Thus RAF1, although essential for the development of skin and lung tumours, is a negative regulator of hepatocarcinogenesis. This unexpected finding highlights the contribution of the cellular/tissue environment in determining the function of a protein, and underscores the importance of understanding the molecular context of a disease to inform therapy design.
Figures

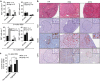

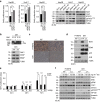
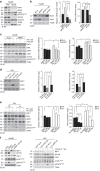
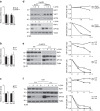
References
-
- El-Serag H. B. Hepatocellular carcinoma. N. Engl. J. Med. 365, 1118–1127 (2011). - PubMed
-
- Pinyol R., Nault J. C., Quetglas I. M., Zucman-Rossi J. & Llovet J. M. Molecular profiling of liver tumors: classification and clinical translation for decision making. Semin. Liver Dis. 34, 363–375 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

