Human Mitochondrial Ferredoxin 1 (FDX1) and Ferredoxin 2 (FDX2) Both Bind Cysteine Desulfurase and Donate Electrons for Iron-Sulfur Cluster Biosynthesis
- PMID: 28001042
- PMCID: PMC5267338
- DOI: 10.1021/acs.biochem.6b00447
Human Mitochondrial Ferredoxin 1 (FDX1) and Ferredoxin 2 (FDX2) Both Bind Cysteine Desulfurase and Donate Electrons for Iron-Sulfur Cluster Biosynthesis
Abstract
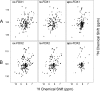
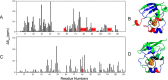
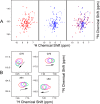
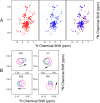
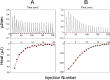
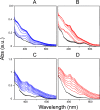
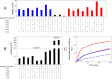
Ferredoxins play an important role as an electron donor in iron-sulfur (Fe-S) cluster biosynthesis. Two ferredoxins, human mitochondrial ferredoxin 1 (FDX1) and human mitochondrial ferredoxin 2 (FDX2), are present in the matrix of human mitochondria. Conflicting results have been reported regarding their respective function in mitochondrial iron-sulfur cluster biogenesis. We report here biophysical studies of the interaction of these two ferredoxins with other proteins involved in mitochondrial iron-sulfur cluster assembly. Results from nuclear magnetic resonance spectroscopy show that both FDX1 and FDX2 (in both their reduced and oxidized states) interact with the protein complex responsible for cluster assembly, which contains cysteine desulfurase (NFS1), ISD11 (also known as LYRM4), and acyl carrier protein (Acp). In all cases, ferredoxin residues close to the Fe-S cluster are involved in the interaction with this complex. Isothermal titration calorimetry results showed that FDX2 binds more tightly to the cysteine desulfurase complex than FDX1 does. The reduced form of each ferredoxin became oxidized in the presence of the cysteine desulfurase complex when l-cysteine was added, leading to its conversion to l-alanine and the generation of sulfide. In an in vitro reaction, the reduced form of each ferredoxin was found to support Fe-S cluster assembly on ISCU; the rate of cluster assembly was faster with FDX2 than with FDX1. Taken together, these results show that both FDX1 and FDX2 can function in Fe-S cluster assembly in vitro.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Architectural Features of Human Mitochondrial Cysteine Desulfurase Complexes from Crosslinking Mass Spectrometry and Small-Angle X-Ray Scattering.Structure. 2018 Aug 7;26(8):1127-1136.e4. doi: 10.1016/j.str.2018.05.017. Epub 2018 Jul 5. Structure. 2018. PMID: 29983374 Free PMC article.
-
The N-Terminus of Iron-Sulfur Cluster Assembly Factor ISD11 Is Crucial for Subcellular Targeting and Interaction with l-Cysteine Desulfurase NFS1.Biochemistry. 2017 Mar 28;56(12):1797-1808. doi: 10.1021/acs.biochem.6b01239. Epub 2017 Mar 17. Biochemistry. 2017. PMID: 28271877
-
Structure of human Fe-S assembly subcomplex reveals unexpected cysteine desulfurase architecture and acyl-ACP-ISD11 interactions.Proc Natl Acad Sci U S A. 2017 Jul 3;114(27):E5325-E5334. doi: 10.1073/pnas.1702849114. Epub 2017 Jun 20. Proc Natl Acad Sci U S A. 2017. PMID: 28634302 Free PMC article.
-
Mechanisms of Mitochondrial Iron-Sulfur Protein Biogenesis.Annu Rev Biochem. 2020 Jun 20;89:471-499. doi: 10.1146/annurev-biochem-013118-111540. Epub 2020 Jan 14. Annu Rev Biochem. 2020. PMID: 31935115 Review.
-
Molecular Details of the Frataxin-Scaffold Interaction during Mitochondrial Fe-S Cluster Assembly.Int J Mol Sci. 2021 Jun 2;22(11):6006. doi: 10.3390/ijms22116006. Int J Mol Sci. 2021. PMID: 34199378 Free PMC article. Review.
Cited by
-
Pan-cancer analyses confirmed the cuproptosis-related gene FDX1 as an immunotherapy predictor and prognostic biomarker.Front Genet. 2022 Aug 5;13:923737. doi: 10.3389/fgene.2022.923737. eCollection 2022. Front Genet. 2022. PMID: 35991547 Free PMC article.
-
Interactions of iron-bound frataxin with ISCU and ferredoxin on the cysteine desulfurase complex leading to Fe-S cluster assembly.J Inorg Biochem. 2018 Jun;183:107-116. doi: 10.1016/j.jinorgbio.2018.03.007. Epub 2018 Mar 15. J Inorg Biochem. 2018. PMID: 29576242 Free PMC article.
-
Architectural Features of Human Mitochondrial Cysteine Desulfurase Complexes from Crosslinking Mass Spectrometry and Small-Angle X-Ray Scattering.Structure. 2018 Aug 7;26(8):1127-1136.e4. doi: 10.1016/j.str.2018.05.017. Epub 2018 Jul 5. Structure. 2018. PMID: 29983374 Free PMC article.
-
Assembly of the [4Fe-4S] cluster of NFU1 requires the coordinated donation of two [2Fe-2S] clusters from the scaffold proteins, ISCU2 and ISCA1.Hum Mol Genet. 2020 Nov 25;29(19):3165-3182. doi: 10.1093/hmg/ddaa172. Hum Mol Genet. 2020. PMID: 32776106 Free PMC article.
-
The role of FDX1 in granulosa cell of Polycystic ovary syndrome (PCOS).BMC Endocr Disord. 2021 Jun 15;21(1):119. doi: 10.1186/s12902-021-00775-w. BMC Endocr Disord. 2021. PMID: 34130686 Free PMC article.
References
-
- Lill R.; Hoffmann B.; Molik S.; Pierik A. J.; Rietzschel N.; Stehling O.; Uzarska M. A.; Webert H.; Wilbrecht C.; Muhlenhoff U. (2012) The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochim. Biophys. Acta, Mol. Cell Res. 1823, 1491–1508. 10.1016/j.bbamcr.2012.05.009. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

