O-Acetyl Side-Chains in Monosaccharides: Redundant NMR Spin-Couplings and Statistical Models for Acetate Ester Conformational Analysis
- PMID: 28001427
- PMCID: PMC5556682
- DOI: 10.1021/acs.jpcb.6b10028
O-Acetyl Side-Chains in Monosaccharides: Redundant NMR Spin-Couplings and Statistical Models for Acetate Ester Conformational Analysis
Abstract
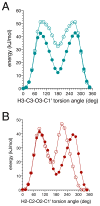
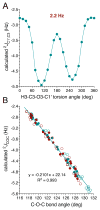
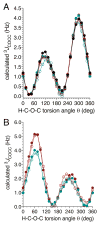
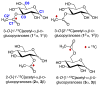
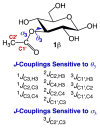
α- and β-d-glucopyranose monoacetates 1-3 were prepared with selective 13C enrichment in the O-acetyl side-chain, and ensembles of 13C-1H and 13C-13C NMR spin-couplings (J-couplings) were measured involving the labeled carbons. Density functional theory (DFT) was applied to a set of model structures to determine which J-couplings are sensitive to rotation of the ester bond θ. Eight J-couplings (1JCC, 2JCH, 2JCC, 3JCH, and 3JCC) were found to be sensitive to θ, and four equations were parametrized to allow quantitative interpretations of experimental J-values. Inspection of J-coupling ensembles in 1-3 showed that O-acetyl side-chain conformation depends on molecular context, with flanking groups playing a dominant role in determining the properties of θ in solution. To quantify these effects, ensembles of J-couplings containing four values were used to determine the precision and accuracy of several 2-parameter statistical models of rotamer distributions across θ in 1-3. The statistical method used to generate these models has been encoded in a newly developed program, MA'AT, which is available for public use. These models were compared to O-acetyl side-chain behavior observed in a representative sample of crystal structures, and in molecular dynamics (MD) simulations of O-acetylated model structures. While the functional form of the model had little effect on the precision of the calculated mean of θ in 1-3, platykurtic models were found to give more precise estimates of the width of the distribution about the mean (expressed as circular standard deviations). Validation of these 2-parameter models to interpret ensembles of redundant J-couplings using the O-acetyl system as a test case enables future extension of the approach to other flexible elements in saccharides, such as glycosidic linkage conformation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures













Similar articles
-
Conformational Populations of β-(1→4) O-Glycosidic Linkages Using Redundant NMR J-Couplings and Circular Statistics.J Phys Chem B. 2017 Apr 13;121(14):3042-3058. doi: 10.1021/acs.jpcb.7b02252. Epub 2017 Mar 30. J Phys Chem B. 2017. PMID: 28296420 Free PMC article.
-
N-Acetyl Side-Chain Conformation in Saccharides: Solution Models Obtained from MA'AT Analysis.J Org Chem. 2022 Jul 1;87(13):8368-8379. doi: 10.1021/acs.joc.2c00189. Epub 2022 Jun 10. J Org Chem. 2022. PMID: 35687878
-
Correlated C-C and C-O bond conformations in saccharide hydroxymethyl groups: parametrization and application of redundant 1H-1H, 13C-1H, and 13C-13C NMR J-couplings.J Am Chem Soc. 2004 Dec 8;126(48):15668-85. doi: 10.1021/ja0306718. J Am Chem Soc. 2004. PMID: 15571389
-
One-Bond 13C-1H and 13C-13C Spin-Coupling Constants as Constraints in MA'AT Analysis of Saccharide Conformation.J Phys Chem B. 2022 Nov 24;126(46):9506-9515. doi: 10.1021/acs.jpcb.2c04986. Epub 2022 Nov 10. J Phys Chem B. 2022. PMID: 36356177
-
A perspective on the primary and three-dimensional structures of carbohydrates.Carbohydr Res. 2013 Aug 30;378:123-32. doi: 10.1016/j.carres.2013.02.005. Epub 2013 Feb 24. Carbohydr Res. 2013. PMID: 23522728 Review.
Cited by
-
Conformational Populations of β-(1→4) O-Glycosidic Linkages Using Redundant NMR J-Couplings and Circular Statistics.J Phys Chem B. 2017 Apr 13;121(14):3042-3058. doi: 10.1021/acs.jpcb.7b02252. Epub 2017 Mar 30. J Phys Chem B. 2017. PMID: 28296420 Free PMC article.
-
Synthesis and O-Glycosidic Linkage Conformational Analysis of 13C-Labeled Oligosaccharide Fragments of an Antifreeze Glycolipid.J Org Chem. 2019 Feb 15;84(4):1706-1724. doi: 10.1021/acs.joc.8b01411. Epub 2019 Jan 29. J Org Chem. 2019. PMID: 30624062 Free PMC article.
-
Nonconventional NMR Spin-Coupling Constants in Oligosaccharide Conformational Modeling: Structural Dependencies Determined from Density Functional Theory Calculations.ACS Omega. 2022 Jul 1;7(27):23950-23966. doi: 10.1021/acsomega.2c02793. eCollection 2022 Jul 12. ACS Omega. 2022. PMID: 35847250 Free PMC article.
-
Reconciling MA'AT and molecular dynamics models of linkage conformation in oligosaccharides.Phys Chem Chem Phys. 2020 Jul 8;22(26):14454-14457. doi: 10.1039/d0cp01389g. Phys Chem Chem Phys. 2020. PMID: 32597425 Free PMC article.
-
Effects of varying the 6-position oxidation state of hexopyranoses: a systematic comparative computational analysis of 48 monosaccharide stereoisomers.J Mol Model. 2017 Jul;23(7):214. doi: 10.1007/s00894-017-3385-x. Epub 2017 Jun 27. J Mol Model. 2017. PMID: 28656484 Free PMC article.
References
-
- Varki A, Lowe JB. Essentials of Glycobiology. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Biological Roles of Glycans. 2. Cold Spring Harbor Laboratory Press; New York: 2009. pp. 75–88. - PubMed
-
- Polacek R, Stenger J, Kaatze U. Chair-Chair Conformational Flexibility, Pseudorotation, and Exocyclic Group Isomerization of Monosaccharides in Water. J Chem Phys. 2002;116:2973–2982.
-
- Rockwell GD, Grindley TB. Effect of Solvation on the Rotation of Hydroxymethyl Groups in Carbohydrates. J Am Chem Soc. 1998;120:10953–10963.
-
- Cloran F, Carmichael I, Serianni AS. Density Functional Calculations on Disaccharide Mimics: Studies of Molecular Geometry and Trans-O-glycosidic 3JCOCH and 3JCOCC Spin-Couplings. J Am Chem Soc. 1999;121:9843–9851.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

