TX-004HR vaginal estradiol has negligible to very low systemic absorption of estradiol
- PMID: 28002201
- PMCID: PMC5404400
- DOI: 10.1097/GME.0000000000000790
TX-004HR vaginal estradiol has negligible to very low systemic absorption of estradiol
Abstract
Objective: To evaluate the pharmacokinetics of TX-004HR vaginal estradiol softgel capsules when used for treating moderate-to-severe dyspareunia in postmenopausal women with vulvar and vaginal atrophy.
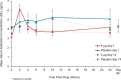
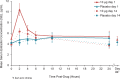
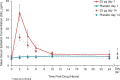
Methods: A substudy of the REJOICE trial (multicenter, double-blind, placebo-controlled, phase 3) evaluated the pharmacokinetics of 4, 10, and 25-μg TX-004HR doses once/d for 2 weeks, followed by twice/wk for 10 weeks. Serum samples obtained at 2, 4, 6, 10, and 24 hours postdose on days 1 and 14, and once on day 84, were analyzed for area under the serum concentration-time curve, tmax, Cmin, Cavg, and Cmax for estradiol, estrone, and estrone conjugates.
Results: Seventy-two women (mean 59 y) participated. TX-004HR 4 μg showed no statistical differences from placebo in estradiol pharmacokinetic (PK) parameters. At 10 μg, estradiol Cmax was statistically higher than placebo on day 1, but was not different from placebo on day 14. With 25 μg, estradiol PK parameters were statistically higher than placebo. Estradiol Cavg values for 25 μg were 9.1 pg/mL on day 1 and 7.1 pg/mL on day 14. Estrone and estrone conjugate PK parameters with TX-004HR were lower than or similar to placebo across all doses. No drug accumulation was observed.
Conclusions: Vaginal TX-004HR resulted in negligible to very low systemic absorption of estradiol. No statistical differences in estradiol PK parameters were observed on day 14 with 4 and 10 μg, and only minor increases were observed with 25 μg (within the normal postmenopausal range). This PK substudy, in conjunction with the primary efficacy results, demonstrated that TX-004HR provided local benefits of estradiol with limited systemic exposure.
Figures
Comment in
-
To the Editor.Menopause. 2017 Aug;24(8):988. doi: 10.1097/GME.0000000000000939. Menopause. 2017. PMID: 28697044 No abstract available.
-
In Reply.Menopause. 2017 Aug;24(8):988-989. doi: 10.1097/GME.0000000000000940. Menopause. 2017. PMID: 28719419 No abstract available.
References
-
- North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20:888–902. - PubMed
-
- Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women's Health Initiative. Maturitas 2004; 49:292–303. - PubMed
-
- The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Committee Opinion No. 659. American College of Obstetricians and Gynecologists. Obstet Gynecol 2016; 127:e93–e96. - PubMed
-
- Ballagh SA. Vaginal hormone therapy for urogenital and menopausal symptoms. Semin Reprod Med 2005; 23:126–140. - PubMed
-
- NAMS Leaders Request Labeling Change for Low-dose Vaginal Estrogen for Vulvovaginal Atrophy. 2016. Available at: https://speakingofwomenshealth.com/news/nams-leaders-request-labeling-ch... Accessed January 6, 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

